- (2014) Volume 15, Issue 4
Vladimir Daoud1, Muhammad Wasif Saif2, Martin Goodman1
1Department of Surgery, and 2Division of Hematology and Oncology, Tufts Medical Center and Tufts University School of Medicine. Boston, MA, USA
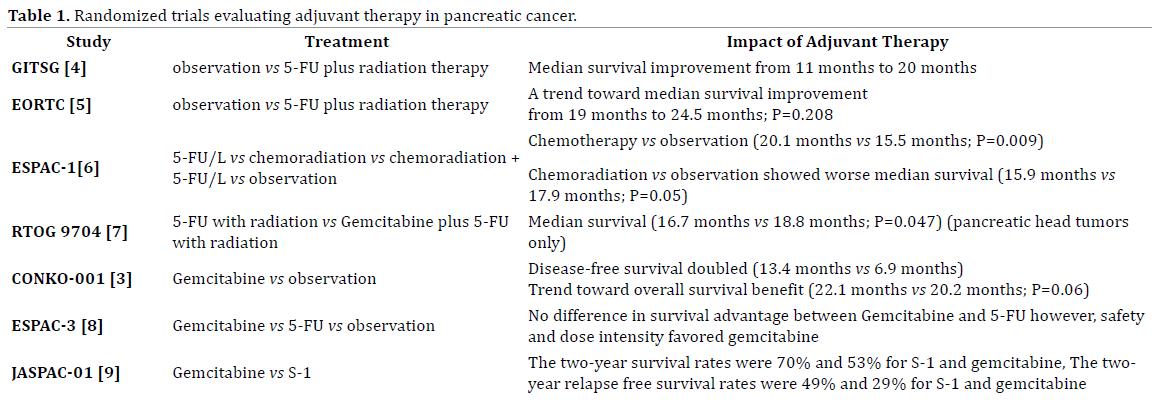
Pancreatic cancer is the fourth leading cause of cancer deaths in both men and women. Surgical resection has been shown to be the only curable treatment available. Unfortunately only 20% of all patients diagnosed with pancreatic cancer are surgical candidates due to the aggressive biology of this disease. There is no clear consensus on what type of adjuvant therapy should be used for patients with pancreatic cancer. Chemoradiation is the favored treatment modality by many in the United States while gemcitabine based chemotherapy is favored in Europe. Both of these approaches have been shown by large prospective, randomized trials to improve disease free intervals and in some studies overall survival. The survival of these patients, even status post resection and adjuvant therapy, remains poor and therefore the need for alternative adjuvant therapies is needed. We will therefore discuss abstracts #4124, #TPS4162, #4120 and #E15191 in this paper which are relevant to the issues described above.
130-nm albumin-bound paclitaxel; Chemotherapy, Adjuvant; gemcitabine; Pancreatic Neoplasms; SLC29A1 protein, human
CONKO: Charité Onkologie; EGFR: epidermal growth factor receptor; EORTC: European Organization of Research and Treatment of Cancer; ESPAC: European Study Group for Pancreatic Cancer; JASPAC: Japanese Adjuvant Study Group of Pancreatic Cancer; RTOG: Radiation Therapy Oncology Group
Venous thromboembolism is the second leading cause of death in cancer patients [1]. The incidence of venous thromboembolism in oncologic patients 3 months after starting chemotherapy isapproximately7% (range 4.6%- 11.6% across cancer locations) and then rising to 13.5% at 12 months (range 9.8%-21.3%) with the highest venous thromboembolism risk associated with pancreatic, stomach, and lung cancer [2]. The incidence of VTE reaches 20% or more in pancreatic cancer and metastatic glioblastoma [3]. Venous thromboembolism is less frequent in Asian patients with ductal pancreatic adenocarcinoma [4]. The genetic and/or epigenetic significance of this finding is currently unknown. As thromboprophylaxis carries a bleeding risk, biomarkers and score predictors were investigated in order to better select the patients who would benefit from prophylactic anticoagulation [5]. Leukocytosis, neutrophilia, monocytosis and thrombocytosis, but not lymphocytosis are all risk factors for thromboembolism in cancer patients [6, 7]. Persistent leukocytosis after first cycle of chemotherapy imparts a 3% risk for venous thromboembolism, as opposed to 1.2% for normal leukocyte counts [8]. Moreover, thrombocytosis is associated with a 2.5 fold risk of venous thromboembolism [9]. A hemoglobin level lower than 10 g/dL and/or the use of erythropoiesis-stimulating agents (ESAs) confer a 1.8- fold increased risk of developing VTE [6]. Contrary to early reports, microparticle-associated tissue factor activity does not appear to play an essential role in the prothrombotic state associated with metastatic pancreatic cancer [10, 11]. Nevertheless, its activity is increased and its small effect in fibrin formation may be part of the multifactorial pathogenesis of venous thromboembolism in the adenocarcinoma of the pancreas [10]. Low-dose warfarin and LMWH are safe for prophylactic anticoagulation in pancreatic cancer [12-14]. Table 1 shows the Khorana and Vienna assessment scores for prediction of venous thromboembolism.
The impact of prothrombotic mutations, PSGL-1 VNTR polymorphism, tissue factor, and soluble P-selectin on venous thromboembolism in cancer patients with adenocarcinoma
Bozkurt et al. evaluated the frequency of inherited [Factor V of Leiden, prothrombin G20210A mutations and PSGL-1 VNRT polymorphisms) and carcinogenesisacquired (Tissue Factor and soluble P-Selectin) protein genotypes and levels respectively in a patients with adenocarcinoma [15]. From a screened population of 1,838 patients with adenocarcinoma, 63 patients with venous thromboembolism and 38 controls had their blood tested for the above mutations and levels. In the population with venous thromboembolism, tissue factor levels were higher in patients with pancreatic adenocarcinoma than those with other tumors (p=0.02). Also, the PSGL-1 VNRT polymorphisms AB and AC were more frequent in central venous catheter related, subclavian, jugular and pelvic venous thromboembolism.
Incidence of incidental and symptomatic venous thromboembolism (VTE) and Khorana’s score in ambulatory pancreatic cancer patients receiving chemotherapy
Muñoz Martin et al. designed a multicentric retrospective cross-sectional study involving a population of ambulatory patients with exocrine pancreatic cancer (EPC) treated with chemotherapy, where the investigators followed 517 patients from January 2008 to December 2011 [16]. Venous thromboembolism was identified in 22.6 % of the patient with a median time to diagnosis of 3 months and 67% of cases occurring within 6 months of diagnosis. Around 50% of the patients had incidental venous thromboembolism. Interestingly visceral thromboembolism was an incidental finding in 91% of the identified cases, representing 38% of all cases of venous thromboembolism in the study. The Khorana score consolidated its clinical usefulness when it predicted symptomatic venous thromboembolism while having no predictive value for incidental venous thromboembolism.
Risk factors for cancer-related venous thromboembolism in ambulatory patients
Cella et al. validated the Khorana score and identified additional predictor factors for venous thromboembolism in their ambulatory oncologic population on antineoplastic therapy [17]. They followed up 544 ambulatory patients with various cancers, 8% of them with pancreatic cancer. Among the confirmed risk factors were previous venous thromboembolism, metastatic disease, vascular or lymphatic compression by the tumor, extremity edema of extremities, surgical procedure in the last 6 months, and central venous catheter. Venous thromboembolism associated with cancer surgery has been increasing frequency although mortality associated with surgery has been decreasing [18]. This new risk factors help to define which population of oncologic patients would benefit most from prophylactic anticoagulation.
Venous thromboembolism is a frequent event in ductal adenocarcinoma. Scores carrying predictive value will support the indication of prophylactic anticoagulation for patients at low bleeding risk and high risk for venous thromboembolism. Biomarkers, especially acquired circulating factors and inherited gene polymorphisms, will redefine venous thromboembolism care in the genomic era. It is now known that Asian patients with ductal adenocarcinoma of the pancreas have lower incidence of venous thromboembolism. It remains to be described if this effect is a consequence of genetics or behaviors (i.e. smoking, metabolic syndrome and diet) affecting epigenetic silencing or activation of cancer pathways. The ESA therapy is one of the components of predicting scores and International Guidelines clearly recommend prophylactic anticoagulation for chemotherapy combinations of thalidomide or lenalidomide and steroids; redefining risks for newly developed monoclonal antibodies and combination chemotherapy for pancreatic cancer and confirming in phase III trials the role of anticoagulation in clinical outcomes will further refine the role of prophylactic anticoagulation in ductal adenocarcinoma.
Authors report no conflict of interest.