Ashwini Kamble1, Praveen Khairkar2*, Ranjana Kale3 and Ramdas Ransingh2
1Department of Biochemistry, Mahatma Gandhi Institute of Medical Sciences, India
2Department of Psychiatry, Mahatma Gandhi Institute of Medical Sciences, India
3Department of Pharmacology, Mahatma Gandhi Institute of Medical Sciences, India
*Corresponding Author:
Praveen Khairkar
Department of Psychiatry
Mahatma Gandhi Institute of Medical Sciences
Sevagram, Maharashtra
India
Tel: +91 7152 284 341
E-mail: Pravinkhairkar@mgims.ac.in
Received date: November 25, 2016; Accepted date: December 30, 2016; Published date: January 05, 2017
Citation: Kamble A, Khairkar P, Kale R, Ransingh R. A Cytochrome 450 Interaction of Chloroquine with Second Generation Antipsychotics. J Pharm Pharm Res. 2017, 1:4.
Copyright: © 2017 Kamble A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Convulsive seizure; Chloroquine; Clozapine; Risperidone; CYP 450
Introduction
Chloroquine is a derivative of 4-aminoquinoline, which is commonly used in the curative and prophylactic treatment for malaria for more than 50 years and its propensity to cause seizure is very rare [1]. Although the literature is replete with neuropsychiatric presentation of chloroquine, there are only few case reports [2-6] which had reported the onset of seizure(s) following chloroquine treatment at therapeutic concentration and overdose. Any schizophrenic patient who does not respond to adequate trials of two or more antipsychotics are them being treated with clozapine. Antipsychotic induced seizures were reported most commonly with clozapine, followed by chlorpromazine and olanzapine, while quetiapine and risperidone were least likely to cause convulsion [7].
Boachie and McGinnity [8] evaluated the largest data on a total of 6344 patients across 10 studies of which 113 patients had clozapine related seizures, at doses ranging between 150 and 600 mg and found that seizure incidence ranged from 0.9% to 29% of clozapine treated patients. But none of their clozapine patients were reportedly simultaneously taking chloroquine and/or later has no mention about it. Chloroquine has found to have potential to interfere in the metabolism of clozapine and risperidone via CYP 450 enzyme system [9]. We would like to discuss a case of possible chloroquine induced grand mal seizures and its CYP 450 enzyme interactions from tertiary rural hospital of central India in patients of schizophrenia, who was stable and in remission while on clozapine 200 mg per day and risperidone 6 mg/day.
Patient and Observation
A 39-year-old right handed carpenter, who had been diagnosed as paranoid schizophrenia of 18 years duration with frequent relapses characterised by suspiciousness, hostility, auditory hallucinations, commenting and commanding type, delusions of control, reference and persecution, poor self-care and marked socio-occupational dysfunction with partial response to multiple trials of chlorpromazine (500 mg/day), olanzapine (20 mg/day), risperidone (12 mg/day) and trifluperazine (20 mg/day). He was finally stabilised with clozapine 200 mg per day and augmentation by risperidone 6 mg/day. He has been maintaining well on same dose for last four years or so and has been on regular follow-up with us. His mean BPRS total and positive symptom subscale scores were reduced significantly. He did not have any past history of seizures, smoking or other substance use or abuse, head injury and any other medical disorder. He had received chloroquine by village health guide; 600 mg on day 1 and 600 mg on day 2 following which he had generalised tonic-clonic seizures which lasted for about a minute or so. He sustained tongue bite injury and passed urine in his cloths. There was history suggestive of postictal confusion and headache lasting for about half an hour and he was rushed to emergency services of our hospital on the same day from his village. He was given intravenous diazepam. His peripheral smear, para-check for malarial antigen and CECT brain were within normal limit, but his EEG was showing generalised high frequency, high amplitude paroxysmal epileptiform discharges (Figure 1). Since patient completed two days of chloroquine physician misinterpreted seizure cause other than chloroquine and advised to take remaining 300 mg of chloroquine on day 3. His clozapine was stopped in view of fever by physician, continued risperidone as it is and psychiatric consultation was suggested. Instead of coming to psychiatric services family members took back the patient home. Second seizure of similar semiology was reported on day 3 about 8 hours after the consumption of chloroquine. This time he was referred to psychiatric services for his drug, his ECG was normal EEG was still showing intermittent, generalized epileptiform activity. He remained hospitalized for observation. Clozapine was restarted, 100 mg per day followed by slow and gradual hike to 200 mg/day. Risperidone continued with earlier 6 mg/day doses. Patient did not have recurrence of seizure in last twelve months.
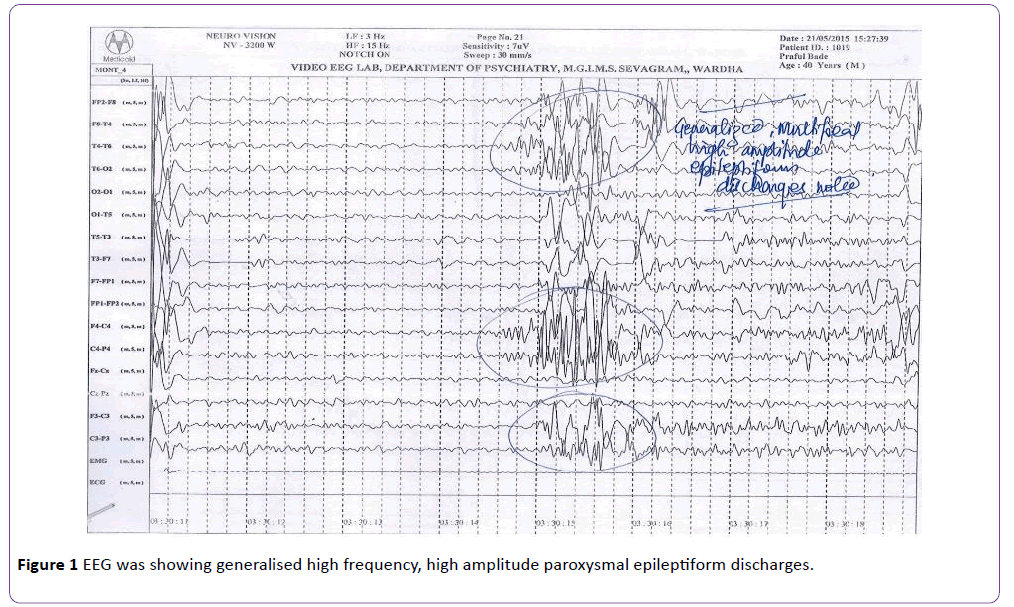
Figure 1: EEG was showing generalised high frequency, high amplitude paroxysmal epileptiform discharges.
Discussion
Considering the rarity of seizure(s) with chloroquine, more likelihood of seizure with clozapine and/or during augmentation of clozapine with risperidone; both being synergistically used in treatment of treatment resistant schizophrenia and the CYP related metabolic interactions among them, we would like to discuss the later perspectives in reference to this case.
Potential seizure(s) with clozapine and to lesser extent risperidone are known [10] but with chloroquine at therapeutic doses they are extremely rare. Indeed since last 50 years have passed and few cases from Nigeria [2], The Netherlands [3], Oman [4], Poland [5], and only two cases from India [6] had reported the occurrence of seizures following therapeutic and intoxicated doses of Chloroquine. According to their studies it could be inferred that seizure may occur within few hours of overdose or between one day and several weeks after the dose of Chloroquine. For example in the study by Bhatia and Malik [6] who reported 30 cases of chloroquine induced neuropsychiatric complications, two of which had grand mal seizure on day 2 and 4. But in both of their patients they observed heralding signs of toxicity like vomiting, diarrhea, abdominal cramps, whereas in our index patient seizure was noted on day 2 and day 3 without any signs of toxicity. Application of Naranjo [11] probability scale indicated that in our index case causal association of chloroquine with seizure is probable (score=6).
One of the interesting studies by Crawley et al. [12] in 2000 suggests that chloroquine does not play role in etiology of seizure in 109 children evaluated for childhood cerebral malaria, although 60% of the later develops seizures. However, distribution studies [13] have shown that chloroquine is extensively sequestered in tissues, the liver, spleen, kidney, and lungs being the main repositories. The concentration of chloroquine within brain is approximately four times greater than plasma [14] and Experimental evidence suggest that chloroquine may precipitates seizure by attenuation of GABA pathways [15] and enhancement of dopaminergic pathway [16], but both of these studies were rather carried out in mice and mechanism in human is not elucidated as yet.
Clozapine is metabolized primarily by CYP1A2, with additional contributions by CYP2C19, CYP2D6 and CYP3A4; while risperidone is metabolized primarily by CYP2D6 and to a lesser extent by CYP3A4 [17]. Two case reports have been published describing patients who experienced a 2-fold increase in serum Clozapine level after taking the CYP2D6 substrate Risperidone [18]. Such increase in Clozapine level is likely due to polymorphism status of CYP2D6. Individuals who are CYP2D6 extensive metabolizers are more susceptible to inhibition of this enzyme [19]. Alfaro et al. [20] showed that addition of risperidone (1 and 2 mg/d) to a clozapine treatment resulted in a strong increase of clozapine plasma levels. Another study by Eap et al. [21] revealed that risperidone at dosages between 2 and 6 mg/d does not appear to significantly inhibit CYP1A2 and CYP2C19 in vivo (median plasma paraxanthine/caffeine ratios before and after risperidone: 0.65, 0.69; p=0.89; median urinary (S)/(R) mephenytoin ratios before and after risperidone: 0.11, 0.12; p=0.75). Although dextromethorphan metabolic ratio is significantly increased by risperidone (median urinary dextromethorphan/dextrorphan ratios before and after risperidone: 0.010, 0.018; p=0.042), risperidone can be considered a weak in vivo CYP2D6 inhibitor, as this increase is modest and none of the eight patients was changed from an extensive to a poor metabolizer. They inferred that increase of clozapine concentrations by risperidone can therefore not be explained by an inhibition of CYP1A2, CYP2D6, CYP2C19 or by any combination of the three.
Following oral or i.v. administration, chloroquine (CQ) is dealkylated into two main metabolites, N-desethylchloroquine (DCQ) and N-bis-desethylchloroquine (BDCQ). At therapeutic concentrations, choloroquine is metabolized into Ndesethylchloroquine (DCQ) primarily via CYP2C8 and CYP3A4. Although CQ possesses cytochrome P450 (P450)-inhibiting properties, notably on CYP2D6, the enzymes catalyzing its Ndealkylation have never been identified [22-23]. Further in chloroquine metabolism, the CYP2C8 and CYP3A4 constitute low-affinity, high-capacity systems, whereas CYP2D6 has a higher affinity but a significantly lower capacity. CQ is well recognized to competitively inhibit CYP2D6 activity in HLM. The inhibitory constant (Ki) values obtained for CQ in HLM (13-15 μM) approximate the Km of CQN-desethylation in recombinant CYP2D6 (19.5 μM), which indicates some affinity for CQ at the CYP2D6 binding site [24]. Results of Projean et al. [25] combined with those obtained in the literature, suggest that CQ may act as a substrate inhibitor of CYP2D6 . This explains that chloroquine has ability to inhibit CYP2D6-mediated metabolism of drugs. Thus those patients who may take chloroquine and are either on clozapine, risperidone or olanzapine which are metabolised through CYP2D6, In Vitro are likely to have transient, but sharp increase in plasma levels of these second generation antipsychotics. Further it can be inferred that chloroquine has potential to raise even the plasma levels of risperidone and more than 6 mg/day of risperidone might then subsequently increase the titre of clozapine which could otherwise not had been possible. Indeed research suggests that inflammation and infection also influence plasma levels of clozapine [26].
CYP2D6 gene is highly polymeric ranging ultrarapid, extensive, intermediate and poor metabolizer phenotypes as stated above. Poor metabolizers and intermediate metabolizers are at risk for dose dependent toxicity. As clozapine and resperidone both are metabolized by CYP2D6 and chloroquine inhibits CYP2D6. Thus it might increase the plasma level of clozapine with possible CYP2D6 polymorphism which might have triggered the seizure activity. So caution is required while prescribing chloroquine in patients who are on anti-psychotics.
Conclusion
Most individuals with schizophrenia take medications indefinitely and at some point take additional medications. Therefore, the risk of polypharmacy related complications is very real in this population. Considering couple of these complex speculations entailing how rapid upward titration of clozapine may increase seizure risk; in our index case who received 6 mg of risperidone along with 200 mg of clozapine, we believe unless patient has had CYP2D6 polymorphism, he would not have seizure related to later. He neither had it earlier when he had been on these combination and stably maintaining better. Why now when no other aggravating factor apart from adding chloroquine in combo of clozapine and risperidone. The question is not easy to answer. This isolated case we believe provides us the unique opportunity to analyse the possibility and advance our insight about how one of the commonly prescribed drug like chloroquine potentially interacts with second generation antipsychotics and possibly can trigger seizure(s).