Research Article - (2023) Volume 9, Issue 3
A Comparative Study on Kinetics Performances of BSA-gold Nanozymes for Nanozyme-mediated Oxidation of 3,3’,5,5’-Tetramethylbenzidine and 3,3’-Diaminobenzidine
Saeed Reza Hormozi Jangi*
Department of Chemistry, Hormozi Laboratory of Chemistry and Biochemistry, Iran
*Correspondence:
Saeed Reza Hormozi Jangi,
Department of Chemistry, Hormozi Laboratory of Chemistry and Biochemistry,
Iran,
Email:
Received: 26-Jun-2023, Manuscript No. IPBMBJ-23-16718;
Editor assigned: 28-Jun-2023, Pre QC No. IPBMBJ-23-16718 (PQ);
Reviewed: 12-Jul-2023, QC No. IPBMBJ-23-16718;
Revised: 17-Jul-2023, Manuscript No. IPBMBJ-23-16718 (R);
Published:
24-Jul-2023, DOI: 10.36648/2471-8084-9.03.21
Abstract
Commonly 3,3'-diaminobenzidine and 3,3',5,5'-thetramethylbenzidine, abbirvated as DAB and TMB, in turn, have
been employed as the standard nanozyme substrates for probing the nanozme-catalyzed oxidation, and the corresponding
oxidation products of these substrates have been probed as the analytical identifiers for sensing/biosensing
application in lab/medial scales. However, it is well-known that the affinity of these substrates toward
binding to enzymes or especially, here, nanozymes is not the same, resulting in different enzyme-like activity and
different kinetics indexes including Km and Vmax. Since, the protein-assisted/protected gold nanozymes (BSA-gold
nanozymes) are type high powerful artificial peroxidase enzymes, Therefore, the kinetics indexes of these nanozymes
including both Km and Vmax should be changed by varying their substrate. To prove this hypotheis, in this
work, a comparative study was performed on the kinetics performances of BSA-gold nanozymes for enzyme-mediated
oxidations of TMB and DAB. The results showed that the Km value of the as-mentioend nanozymes was 0.03
mM and 0.72 mM toward TMB and DAB, in order, revealing that the affinity of TMB for binding to the nanozyme
active nodes is significantly higher than its alternative substrate, DAB due to its lower Km value. In contrast, the Vmax of the enzymatic reaction was found to be 263 nM sec-1 and 185 nM sec-1 for the nanozmye-mediated oxidation of
TMB and DAB, respectively. The higher Vmax of the nanozyme-mediated oxidation of TMB revealed that the catalytic
efficiency of the as-mentioend nanozymes toward TMB oxidation is characterstically higher (about 1.5-fold) than
that of the DAB oxidation. The difference between the kinetic indexes of TMB and DAB may be related to their
different oxidation pathways and their different reactivity. In fact, the DAB oxidizes via an n-electron irreversible
oxidation pathway to produce an indamine polymer. While TMB nanozyme-mediated oxidation has occurred upon
a 2-electron reversible mechanism for the production of a cation radical. These different pathways resulted in different
kinetic performances.
Keywords
Kinetics performances; BSA-Gold nanozymes; 3,3',5,5'-thetramethylbenzidine; 3,3'-diaminobenzidine
Introduction
It is well known that although native enzymes reveal high specificity
toward their substrates and high catalytic performance, they
show several disadvantages such as low stability (narrow pH and
thermal range); difficult recovery, and no reusability. For overcoming
these drawbacks, the development of enzyme immobilization
processes has been considered as a reliable way. The enzyme immobilization
permits possible increase in stability (pH, thermal,
storage, and solvent performance), easy to recovery and handling
via simple enzyme separation from the mixtures of reactants and
products. Despite great advantages, the specific and relative activities
of the most immobilized enzymes are found to be lower
than the free enzymes. This decrease in the enzyme activity can
be explained by the effect of immobilization on enzymes' conformational
transition after their immobilization. To avoid the difficult of the enzyme immobilization and for solving its significant drawbacks
especially, the enzyme inactivation during the immobilization
process, development of artificial enzymes or enzyme mimics
is considered. By fast development of nanotechnology, a new approach
for developing artificial enzymes is developed by utilizing
highly stable nanoparticles with high enzyme-like activity in the
enzyme-catalyzed reactions as the artificial enzymes which commonly
called as nanozymes. On the hand, the significant progress
of nanochemistry and material science in recent years open a new
door for design and development of the high-performance and
novel nano-supports toward different applications, for instance,
MOFs, catalytic materials, and nanoparticles with enzyme-like activity
[1-14].
Among different artificial enzymes, nanozymes with very high enzyme-
like activity and ultra-stability are recently considered the
first and the best choice for proceeding enzyme-catalyzed reactions
with higher efficiency compared to native enzymes. There
are several different practical and medical applications for nanozymes,
as reported in the literature, for instance, they have been
utilized for the analytical sensing/biosensing of a verity of species.
Besides, the nanozymes were used as reusable catalysts with high
catalytic efficiency for biocatalysis of reactions instead of natural
enzymes for instance, using in enzyme-mediated dye degradation,
water treatment, food quality checking and etc [1,5,15-17].
The first report on the enzyme-like nanomaterials was in 2007 by
Gao et al. which introduced the iron oxide nanoparticles as the
peroxidase mimicking materials with high catalytic efficiency and
characteristic stability. After the first report of Prof. Gao as the pioneer
and founder of the nanozyme field in sensing, detection,
and catalysis, various nanomaterials such as noble metals, metal
oxides, and carbon materials were introduced as enzyme mimics
(nanozymes) [5].
Various nanomaterials exhibit peroxidase-like activity, hence, most
of the nanozymes are peroxidase mimetics. For instance, there
several reports on the peroxidase like activity of MnO2 nanoparticles,
bismuth oxyiodide nanoflowers (abbreviated as BiOI-NFs),
different types (modified or unmodified) of silver nanoparticles
[20], protein-assisted/protected gold nanoclusters (e.g., BSAAu
nanoclusters), SiO2-Fe3O4 NPs, and some novel metal-organic
frameworks (MOFs), e.g., NEQC-340 [18-24].
Due to the significant peroxidase-like activity of majority of nanozymes,
these compounds can be used for designing catalyst-based
analytical sensors, called nanozyme-based sensors. Up to now, several
nanozyme-based sensors have been constructed for the quantification
of a variety of analytes such as cysteine, hydrogen peroxide,
speciation of mercury(II) ions, filed-detection of explosive,
triacetone triperoxide, enzyme-free sensing of glucose, and glutathione
bioquantification. The majority of the nanozyme-based
sensors are based on probing the nanozyme-catalyzed oxidation
of 3, 3´, 5, 5´-tetramethylbenzidine (TMB) to blue cation radical.
However, in 2020, Hormozi Jangi et al. introduced a series of nanozyme-
based sensors using the n-electron irreversible oxidation of
3,3′-diaminobenzidine (DAB) to a stable brown colored indamine
polymer rather than common cation radicals resulting from TMB.
Moreover, a novel class of nanozyme-based sensors called multinanozyme
sensor was also developed by Hormozi Jangi et al. toward
enhancing both sensitivity and selectivity of the sensor via the synergetic effect of multinanozyme system on the catalytic activity and specificity [5,15,16,18,23-28].
Recently catalytic property of gold nanozymes and their excellent
peroxidase-like activity brought them up as safe, green, and
high throughput alternatives of natural peroxidase enzymes to
developed enzyme-mediated sensors. As we mentioned previously,
commonly TMB and DAB have been applied as nanozyme
substrates for probing the nanozme-catalyzed oxidation, and the
corresponding oxidation products of these substrates have been
probed as the analytical identifiers for sensing aims. However, it
is well-known that the affinity of TMB and DAB substrates toward
binding to enzymes or especially, here, nanozymes is not the same,
resulting in different enzyme-like activity and different kinetics indexes
including Km and Vmax. Since, the protein-assisted/protected
gold nanozymes (BSA-gold nanozymes) are a type a high powerful
artificial peroxidase enzymes, Therefore, the kinetics indexes including
both Km and Vmax of these nanozymes should also be varied
by varying their substrate [9,11,13,21-24,27].
Considering the above considerations, in this study, we aimed
to perform a comparative study on the kinetics performances of
protein-assisted/protected gold nanozymes for nanozyme/enzyme-
mediated oxidation of TMB and DAB. To do this, initially,
protein-assisted/protected gold nanozymes were synthesized via a protein-directed method utilizing Bovine Serum Albumin (BSA)
as both reductant and stabilizer. Thereafter, the as-prepared nanozymes
were characterized by recording their TEM images and DLS
pattern of these nanozymes. Thereafter, the Michaelis-Menten
model and the Lineweaver-Burk method were utilized for the calculation
of kinetic parameters of BSA-gold including Km and Vmax for
both DAB and TMB as the chromogen substrates.
Materials and Methods
Materials
DAB was obtained from Sigma Aldrich Company. Deionized water
was obtained from Zolal Teb chemical company (Iran). Other materials
were obtained from Merck Company in their analytical or
synthesis grades.
Instrumentations
The UV-Visible measurements for the investigation of the peroxidase-
like activity of the as-prepared nanozymes as well as for
probing nanozyme activity toward oxidation of both DAB and TMB
by using an Ultrospec 4000 UV-Vis spectrophotometer manufactured
by Pharmacia Biotech (Biochrom) Ltd equipped with SWIFT
Software. Besides, a Metrohm 827 pH meter equipped with a
combined glass electrode and a transmission electron microscope
(Zeiss, model EL10C) were utilized for pH measurements and size
and morphology evaluation, in order. For exploring more precise
on size reporting, the DLS pattern was provided using a Shimadzu
particle size analyzer (model: SALD-301V, Japan).
Green Synthesis of BSA-Gold Nanozymes
Protein protected-gold nanozymes were synthesized via a simple,
high throughput and green method at physiological temperature
via incubation of 10 mL mixed solution (pH=10.5-11.0) of a 1:1
volume ratio of HAuCl4.4H2O solution and 50 mg mL-1 at 37.0°C
for 12 hours. The as-prepared nanozymes were then collected and
stored at 4°C [21].
Nanozyme Activity Assay and Kinetics Studies
For the DAB assay, 80.0 μL BSA-gold nanozymes were added into
1.3 mL of phosphate buffer solution (pH 7.0, 0.4 M) containing
200.0 μL of DAB (different concentrations) and 40.0 μL of HP (with
a final concentration of 0.24 M) and thoroughly mixed at ambient
temperature. The oxidation was followed for 25.0 min to complete
the production of the corresponding indamine polymer (i.e., poly-
DAB). Thereafter, the nanozyme activity (nM sec-1) was measured
by probing the absorbance of the resulting polyDAB at 460 nm
considering a molecular extinction coefficient ɛ=5500 molar cm-1.
Regarding the TMB assay, 40 μL hydrogen peroxide solution (final
concentrations of 0.24 M), 200 μL of TMB (different concentrations),
and 80 μL of BSA-gold nanozymes were introduced to 1.3
mL of acetate buffer (0.3 M; pH, 0.4), followed by incubation for
about 10 minutes at ambient temperature. After that, the absorbance
of the blue-colored TMB-ox at 658 nm was used for nanozyme
activity (nM sec-1) calculation considering a ɛ of 39000 M
cm-1. Finally, the kinetic parameters of the as-prepared BSA-gold
nanozymes for both DAB and TMB were investigated based on
Michaelis-Menten equation and the Lineweaver-Burk method as
the standard models for evaluation of the enzyme kinetics performances.
Results and Discussion
Characterization of as-Mentioned Nanozymes
Protein-assisted/protected gold nanozymes called as BSA-gold nanozymes were synthesized by using the native bovine serum albumin (BSA) as both reductant and stabilizer in physiological temperature and basic pH utilizing a protein-directed method as a green, high throughput, and one-pot approach. The morphological characterization of the as-prepared nanozymes was performed via recording their TEM image with a transmission electron microscope. The results of this analysis are shown in Figure 1, the as-prepared BSA-gold nanozymes reveal semi-spherical morphological properties with an approximately narrow size distribution which makes them suitable for enzyme-mimicking applications. More precisely, the results showed that the as-mentioned nanozymes have a very narrow size distribution over 7.7 nm-18.3 nm. The narrow size distribution of these nanozyme can be considered as a significant advantage for them because the variation of the size of nanozymes can significantly affect their enzymatic activity, for instance, increasing the size of the nanozymes leads to decreasing the active surface area. Consequently their active enzyme- like nodes for binding the enzyme substrate have decreased and the enzyme-like activity of the nanozymes was decreased, as previously reported. It is notable that the mean size of the as-prepared nanozymes was estimated at about 13.2 nm [21] (Figure 1).
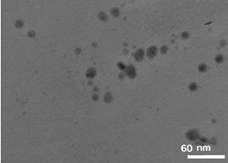
Figure 1: TEM image of as-prepared BSA-gold nanozymes
However, for exploring more precise size reporting of the as-mentioned nanozymes, the DLS analysis of the nanozymes was performed. The results are shown in Figure 2, as shown, the as-mentioned nanozymes show a size distribution over 7.3 nm-31.3 nm with an average diameter of 13.16 nm. However, the maximum of particles has a size in the range of 10.5 nm-17.2 nm. Besides, the mode of the particles has a size of about 13.0 nm. It is notable that the results obtained from the DLS analysis (mean size of about 13.16 nm) are close to those provided from the TEM imaging method (mean size of about 13.2 nm) (Figure 2).
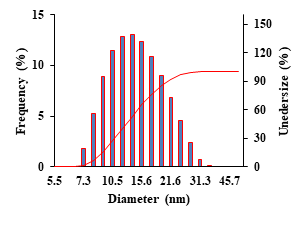
Figure 2: DLS analysis of as-mentioned BSA-gold nanozymes
Proving Nanozymatic Activity of the as-Prepared BSA-Gold Nanozymes
The nanozymatic (peroxidase-like) activity of the as-synthesized BSA-gold nanozymes was evaluated by nanozyme-catalyzed 3,3',5,5'-tetramethylbenzidine (TMB) oxidation. The activity was evaluated by probing the absorbance of the blue-colored oxidation product (i.e., TMB-ox) at 655 nm based on the standard enzyme assay. It is notable that to prove the oxidation catalysis by the as-prepared nanozymes, the oxidation process was performed in the presence and the absence of the as-prepared nanozymes. The results are shown in Figure 3, as seen in this figure, the oxidation of TMB to its corresponding blue-colored product cannot efficiently proceed in the absence of the as-prepared nanozymes, revealing very slow kinetics of the oxidation process of TMB by hydrogen peroxide. In contrast by introducing the as-prepared nanozymes into the mixture of TMB and hydrogen peroxide, the oxidation quickly proceeded and a significant absorbance was observed at 658 nm which is related to the oxidation product of TMB. Based on these results, it can be concluded that the as-prepared BSA-Au nanozymes have a great nanozymatic activity (Figure 3) compared to the control sample.
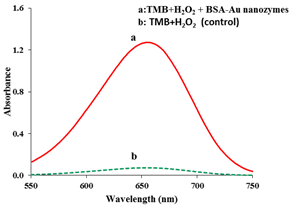
Figure 3: Nanozymatic activity of the as-prepared BSA-Au nanozymes compared to the control sample
Kinetics Parameters of BSA-gold nanozyme for TMB Oxidation
To explore more precise on the peroxidase-like activity and enzymatic power of the as-prepared nanozymes, the kinetics studies were carried out by estimating the nanozyme activity as a function of TMB concentration and then estimating the nanozymatic kinetics parameters, the standard Lineweaver–Burk plot was provided by plotting the inverse of the velocity of the nanozymatic reaction (V-1) as a function of [TMB]-1. It is notable that the velocity of the reaction was calculated based on the estimation of the concertation of the produced TMB-ox per one second of the reaction (μM sec-1), considering this fact that the molar absorption efficient of TMB-ox at 655 nm was 39000 μM-1 cm-1. The kinetic parameters of the as-mentioned nanozymes including Vmax and Km were then calculated by plotting the steady-state Michaelis-Menten curve and the linear plot of Lineweaver-Burk for both substrates.The Michaelis-Menten plot for the enzymatic oxidation of TMB catalyzed by the as-mentioned nanozymes was shown in Figure 4A, revealing that the rate of nanozyme-mediated oxidation of TMB was increased by increasing the substrate concertation and then leveled off. To estimate the kinetic parameters of BSA-gold nanozymes toward TMB oxidation, the Lineweaver–Burk plot was constructed (Figure 4B).Considering the results obtained in Figure 3B, the Vmax and Km of the as-mentioned nanoymes toward TMB oxidation were calculated at about 263 nM sec-1 and 0.03 mM, in order. The high value of the Vmax of the as-prepared nanozymes for TMB oxidation compared to the Vmax of the natural horseradish peroxidase (Vmax=20.1 nM sec-1) revealed that the as-prepared nanozymes have a greater catalytic efficiency toward the oxidation of TMB compared of the natural enzyme which make them as high powerful alternative for the enzyme [29]. Besides, the very lower Km of these nanozymes compared the natural enzyme (Km=0.43 mM) reveal that the very higher affinity of the TMB toward the as-prepared nanozymes compared to the HRP enzyme. In fact, the ratio of Vmax (nanozyme/Vmax (HRP)) was calculated at about 13, revealed that the catalytic efficiency of the as-mentioned nanozymes for TMB oxidation. It is 13-fold higher than that of natural enzyme [2-4,29] (Figure 4).
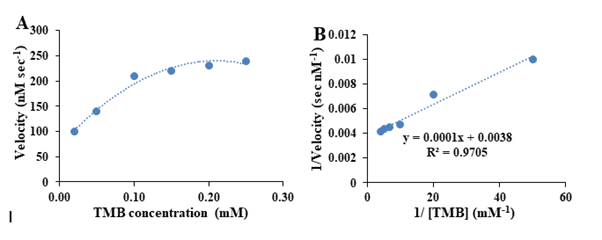
Figure 4: Kinetic performances of BSA-Au nanozymes toward TMB oxidation, (A) Michaelis-Menten plot and (B) Lineweaver-Burk plot
Kinetics Parameters of BSA-gold Nanozyme for DAB oxidation
To evaluate the kinetics performances of the as-mentioned BSAgold nanozymes for the enzyme-catalyzed DAB oxidation, the steady-state Michaelis–Menten plot was constructed for the DAB oxidation by hydrogen peroxide in the presence of the as-mentioned nanozymes as the peroxidase-mimicking agents, and the results are represented in Figure 5A, revealed that the rate of nanozymatic DAB oxidation was increased by increasing the substrate concertation and then leveled off which is same of the TMB oxidation but the TMB oxidation rate was found to be higher than that of the DAB oxidation by the as-mentioned nanozymes. To explore more precise on the calculation of the kinetic indexes of the BSA-gold nanozymes toward DAB oxidation, the linear Lineweaver- Burk plot was constructed for the more accurate estimation of Km and Vmax of BSA-gold enzymes-mediated oxidation of DAB (Figure 5B), revealing a Vmax and Km of 185 nM sec-1 and 0.72 mM, in order, for the DAB oxidation. The value of the Vmax of the as-prepared nanozymes for DAB oxidation was found to be higher than that of the natural, revealing its greater catalytic efficiency compared of the natural enzyme (Figure 5).
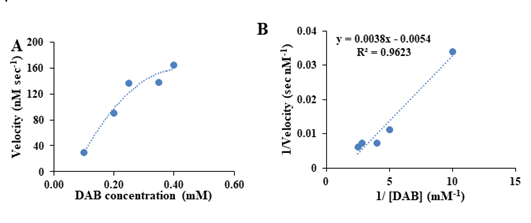
Figure 5: Kinetic performances of BSA-Au nanozymes toward DAB oxidation, (A) Michaelis-Menten plot and (B) Lineweaver-Burk plot
Comparison of Kinetic Performances of BSA-Gold Nanozymes for Different Substrates
Table 1 shows the kinetic parameters of the as-mentioned BSAgold nanozymes including Km and Vmax for the nanozyme-medaited oxidation of both DAB and TMB, as seen in this table, the Vmax of DAB oxidation was found to be lower than that of the TMB oxidation which pointed to the fact that the catalytic efficiency of the as-mentioned nanozymes toward TMB is significantly higher than their efficiency for the DAB oxidation. The ratio of Vmax (TMB)/Vmax (DAB) was estimated as high as 1.42, revealing that the catalytic efficiency of the as-prepared BSA-gold nanozymes for TMB oxidation is about 1.5-fold higher than their catalytic performances for the DAB oxidation. The difference between the kinetic indexes of TMB and DAB may be related to their different oxidation pathways and their different reactivity. In fact, the DAB oxidizes via an n-electron irreversible oxidation pathway to produce an indamine polymer. While TMB nanozyme-mediated oxidation has occurred upon a 2-electron reversible mechanism for the production of a cation radical. These different pathways resulted in different kinetic performances. Besides, the Km value for DAB was found to be 24-fold higher than that for TMB. It is well-known that the Km shows the affinity of an enzyme to its substrate which its lower value assigned to a higher affinity [2-4]. Hence, the very higher Km for DAB reveals the very lower affinity of DAB for binding to BSAgold nanozyme active nodes compared to its alternative substrate, TMB. This difference can be related to the different reactivity of DAB and TMB. For exploring more precise on the different affinity and reaction kinetics of the nanozyme-mediated oxidation of TMB and DAB, the schematic representation of TMB oxidation pathway compared of DAB oxidation mechanism on the surface of BSA-gold nanozyme was provided and represented in Scheme 1. As can be seen from this scheme, the TMB oxidation on the surface of the as-prepared nanozymes occurs via a reversible two-electron oxidation pathway by the produced active hydroxyl radicals generated form reaction of hydrogen peroxide over the as-mentioned nanozymes, as previously reported. In contrast, the oxidation of DAB occurs on the surface of the as-mentioned nanozymes by reacting the DAB molecule with hydroxyl radicals, followed by successive polymerization to produce an indamine polymer via an irreversible n-electron pathway. Considering these facts and different reactivity of TMB and DAB, the difference between the kinetics indexes of BSA-gold nanozymes for their oxidation is clearly acceptable [2-5,8,23,24,27] (Table 1) (Figure 6).
Table 1: Kinetic parameters of BSA-gold nanozymes for TMB oxidation compared to those for DAB oxidation.
| Substrate |
Oxidation pathway |
Km (mM) |
Vmax (nM sec-1) |
| DAB |
n-electron irreversible |
0.72 |
185 |
| TMB |
2-electron reversible |
0.03 |
263 |
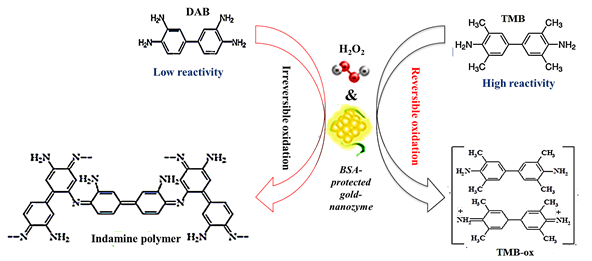
Figure 6: DAB oxidation pathway against TMB oxidation pathway on surface of BSA-gold nanozymes in the presence of hydrogen peroxide as oxidizing agent
Conclusion
Herein, a comparative study was performed on the kinetics performances
of BSA-gold nanozymes for enzyme-mediated oxidations
of 3,3',5,5'-thetramethylbenzidine, and 3,3'-diaminobenzidine.
The results showed that the Km value of BSA-gold nanozymes was
0.03 mM and 0.72 mM toward TMB and DAB, in order, to reveal
the higher affinity (lower Km) of TMB for binding to nanozyme
active nodes compared to its alternative substrate, DAB. In contrast,
the Vmax was found to be 263 nM sec-1 and 185 nM sec-1 for
nanozmye-mediated oxidation of TMB and DAB, respectively. The
higher Vmax of the nanozyme-mediated oxidation of TMB revealed
that the catalytic efficiency of BSA-Au nanozymes toward TMB oxidation
is higher (about 1.5-fold) than that of the DAB oxidation.
The difference between the kinetic indexes of TMB and DAB may
be related to their different oxidation pathways and their different
reactivity. In fact, the DAB oxidizes via an n-electron irreversible
oxidation pathway to produce an indamine polymer. While TMB
nanozyme-mediated oxidation has occurred upon a 2-electron reversible
mechanism for the production of a cation radical. These
different pathways resulted in different kinetic performances.
Acknowledgment
The authors gratefully thank the Hormozi Laboratory of Chemistry
and Biochemistry for the support of this work.
Conflict Of Interest
None.
References
- Zhou Y, Liu B, Yang R, Liu J (2017) Filling in the gaps between nanozymes and enzymes: Challenges and opportunities. Bioconjug Chem. 28(12): 2903-2909.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M (2021) High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochem. 105(3): 79-90.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M (2022) Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochem. 120: 138-155.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M, Dehghani Z (2020) High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochem. 90(11): 102-112.
[Crossref] [Google Scholar]
- Jangi ARH, Jangi MRH, Jangi SRH (2020) Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chin J Chem Eng. 28(6): 1492-1503.
[Crossref] [Google Scholar]
- Jangi SRH (2023) Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chem Res Nano mat. 1(4): 1-9.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M (2020) High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. J Chem Sci. 132: 1-8.
[Crossref] [Google Scholar]
- Jangi SRH (2023) Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: A new approach toward reuse instead of common mineralization. Chem Pap. 1-15.
[Crossref] [Google Scholar]
- Jangi SRH, Dehghani Z (2023) Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chem Res Nano Mat. 2(1): 15-23
[Crossref] [Google Scholar]
- Zeng X, Ruan Y, Chen Q, Yan S, Huang W (2023) Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chem Eng J. 452: 138422.
[Crossref] [Google Scholar]
- Jangi SRH (2023) Evaluating the effect of shelf-storage, daylight, and air oxygen on the peroxidase-like activity of unmodified silver nanoparticles.
[Crossref] [Google Scholar]
- Yang X, Xiang J, Su W, Guo J, Deng J, et al. (2023) Modulating Pt nanozyme by using isolated cobalt atoms to enhance catalytic activity for alleviating osteoarthritis. Nano Today. 49: 101809.
[Crossref] [Google Scholar]
- Jangi SRH (2023) Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M (2021) Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sens Actuators B Chem. 339: 129901.
[Crossref] [Google Scholar]
- Chen X, Wang Y, Feng M, Deng D, Xie X, et al. (2023) Dual-active-site Fe/Cu single-atom nanozymes with multifunctional specific peroxidase-like properties for S2- detection and dye degradation. Chin Chem Lett. 34(6): 107969.
[Crossref] [Google Scholar]
- Bittencourt GA, Vandenberghe LPD, MartÃnez-Burgos WJ, Valladares-Diestra KK, de Mello AFM, et al. (2023) Emerging contaminants bioremediation by enzyme and nanozyme-based processes-a review. Iscience. 26: 106785.
[Crossref] [Google Scholar]
- Li Z, Hu J, Zhan Y, Shao Z, Gao M, et al. (2023) coupling bifunctional nanozyme-mediated catalytic signal amplification and label-free sers with immunoassays for ultrasensitive detection of pathogens in milk samples. Anal Chem. 95(15): 6417-6424.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M, Absalan G (2020) A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3'-diaminobenzidine. Mikrochim Acta. 187: 431.
[Crossref] [Google Scholar]
- Ahmadi-Leilakouhi B, Jangi SRH, Khorshidi A (2023) Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chem Pap. 77(2): 1033-1046.
[Crossref] [Google Scholar]
- Geng X, Xue R, Liang F, Liu Y, Wang Y, et al. (2023) Synergistic effect of silver nanoclusters and graphene oxide on visible light-driven oxidase-like activity: Construction of a sustainable nanozyme for total antioxidant capacity detection. Talanta. 259: 124565.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M, Absalan G (2020) A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Anal Chim Acta. 1127(6): 1-8.
[Crossref] [Google Scholar]
- Akhond M, Jangi SRH, Barzegar S, Absalan G (2020) Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chem Pap. 74: 1321-1330.
[Crossref] [Google Scholar]
- Jangi SRH, Davoudli HK, Delshad Y, Jangi MRH, Jangi ARH (2020) A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surf Interfaces. 21: 100771.
[Crossref] [Google Scholar]
- Jangi SRH, Akhond M (2020) Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchem J. 158: 105328.
[Crossref] [Google Scholar]
- Singh AK, Bijalwan K, Kaushal N, Kumari A, Saha A, Indra A (2023) Oxidase-like nanozyme activity of manganese metal-organic framework nanosheets for colorimetric and fluorescence sensing of l-cysteine. ACS Appl Nano Mater. 6(9): 8036-8045
[Crossref] [Google Scholar]
- Li T, Wang Y, Liu W, Fei H, Guo C, Wei H (2023) Nanoconfinement-guided construction of nanozymes for determining H2O2 produced by sonication. Angew Chem Int Ed. 62(12): e202212438.
[Crossref] [Google Scholar]
- Zhang K, Luo M, Rao H, Liu H, Li J, et al. (2023) Integrating plasmonic and nanozyme responses of gold nanoparticles for enhancing photothermometric sensing. Sens Actuators B Chem. 392: 134067.
[Crossref] [Google Scholar]
- Chen J, Liu X, Zheng G, Feng W, Wang P, et al. (2023) Detection of glucose based on noble metal nanozymes: Mechanism, activity regulation, and enantioselective recognition. Small. 19(8): 2205924.
[Crossref] [Google Scholar]
- Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, et al. (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2(9): 577-583.
[Crossref] [Google Scholar]
Citation: Hormozi Jangi SR (2023) A Comparative Study on Kinetics Performances of BSA-gold Nanozymes for Nanozyme-mediated Oxidation of 3,3',5,5'-Tetramethylbenzidine and 3,3'-Diaminobenzidine. Biochem Mol Biol J. 9:21.
Copyright: © 2023 Hormozi Jangi SR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.







