Keywords
Mesotherapy, Rejuvenation, Facial revitalization, Cell extracts, Anti-aging.
Introduction
Anti-aging techniques and procedures are gaining more and more interest in the modern society. Apart from the internal organic and systemic rejuvenation, facial revitalization is an essential part of holistic rejuvenation. Process of facial aging is associated with facial skin volume loss, reduction of the elasticity, moistness and thickness, appearance of wrinkles and folds, pigmented spots, lentigos, and keratosis.[1-3] Histological changes include thinning of epidermis, flattening of epidermal-dermal interface, reduction and disorganization of collagen and elastin, slowdown of cell turnover, reduction of melanocytes and their erratic activity and the phenomenon of solar elastosis.[4-7]
Age-related changes in the dermis are the results of intrinsic factors (genetics and life-style) and continuous environmental damage, predominantly ultraviolet radiation, which is responsible for skin’s photo-aging.[8,9] Degeneration of the dermal connective tissue leading to reduction of the extracellular matrix is responsible for the wrinkling and sagging of the skin. It has being proven by multiple studies that aging process affects synthesis and remodeling of the main extracellular matrix components in the skin, namely collagen and elastin. It results in reduction of the extracellular matrix density and affects its quality.[10,11] The traditional approach to facial rejuvenation focuses on various dermatologic cosmetic procedures, i.e., laser resurfacing, microdermabrasion, and electric stimulation. These procedures mainly target the collagen production by the fibroblasts in the derma. However, in recent years there has been a shift in the anti-aging strategies towards increasing significance of mesotherapy.[12-14]
Mesotherapy was initially introduced as intra dermal injections of pharmacological substances directly into the treated area by Michel Pistor in 1940’s and 50’s. Although mesotherapy was well researched and widely used since 1958 it was officially recognized as a medical specialty only in 1987. Intradermotherapy was referred to as intradermal injection of highly diluted drugs. In 1976, Michel Pistor himself described mesotherapy technique as follows: “a little volume, a few times, and in the right place”.[15] The list of biologically active substances used for mesotherapy is vast and its mechanisms of action greatly vary depending on the injected products. Most of the described techniques involve intradermal injections of mixture of various products, usually vitamins, amino acids, nucleotides, coenzymes, and hyaluronic acid.[16-18]
The ultimate goal of mesotherapy in skin rejuvenation is restoration and maintenance of healthy and young-looking skin. The targeted effects include improvements of skin elasticity, firmness, hydration, and pigmentation and are achieved by injection into the superficial dermis of biocompatible and absorbable products. A number of clinical studies has shown some of mesotherapy products promoting fibroblast activation and stimulation of collagen biosynthesis.[19-21] However, most of the available data is inconsistent. Another concern is possible complications, among which the most frequently reported are: mycobacterial infection, scarring, formation of hard lumps in the area of injection, lichenoid keratosis, cutaneous necrosis, etc.[22]
It is absolutely clear now that there is no perfect universal product or mixture of substances that would guarantee best results with the minimal risk of complications. Our approach to facial rejuvenation is based on stimulating the regenerative processes in the skin by the means of paracrine effect as well as stimulation of endogenous synthesis of extracellular matrix components.
Hence, the objectives of this study were to evaluate the efficacy of mesotherapy with a cocktail containing combination of cell extracts from skin, placenta and mesenchyme with addition of collagen and elastin proteins in rejuvenation and revitalization of the facial skin.
Materials and Methods
The observed cohort comprised of twentysix healthy women in the age of 40-65 y.o. (mean age 56 ± 2.5) with Fitzpatrick skin types III and IV and Glogau score II-III. Exclusion criteria were pregnancy, breastfeeding, and women who were under medical treatment for acute illness. Treatment consisted of mesotherapy applied on the forehead, cheeks, periorbital and perioral areas, chin and neck with a 33-34G needle. The injected formulation was MF+ Mito Organelles™ SPMCE (MF+ MO™ SPMCE) – peptides extracted from skin, placenta, mesenchyme, with addition of collagen and elastin. Treatment sessions were performed once per week during 12 weeks.
Results were assessed 1 and 3 months after the last mesotherapy session using photographic comparison, patient’s subjective view and external dermatologist expert’s opinion, clinical and instrumental evaluation, and histopathology report. The photographic evaluation of skin conditions were done before the treatment, every two weeks, in the end of the treatment, one month and three months after the treatment protocol completed.
Rating was based on a 5-point scale on which improvement was rated as none (0%), mild (1–25%), moderate (26–50%), good (51– 75%), or excellent (76–100%).
The following instrumental methods of evaluation were performed: hydration, elasticity and pigmentation/melanin level by “Soft Plus” device (Callegari S.P.A., Italy). Photographic evaluation of wrinkles, including its 3D analysis and profilometry was done using the “Soft Plus” device (Callegari S.P.A., Italy) and “Visioface” (Germany).[23-26]
Changes of the skin roughness were assessed according to the Glogau score throughout the treatment protocol, one and three months post-treatment.[27]
Biopsies of the facial skin were taken prior to commencement of treatment and three month post-treatment. Punch biopsies taken three months after the treatment protocol were obtained from the immediate proximity to the one of the pretreatment. The biopsy material was sent for further histological analysis and immunohistochemical staining. Quantitative evaluation of collagen types I, III, and VII, newly synthesized collagen, total elastin, and tropoelastin was performed using a morphometric analysis. Content of collagen types I and III, and elastin were measured using immunoperoxidase method. Collagen type VII and propoelastin were determined using immunofluorescence staining.[28,29]
Results and Discussion
There were two minor adverse reactions observed during the trial. In both cases ladies had mild to moderate transitory edema in the periorbital area after the third session of mesotherapy. The edema subsided within 2-3 days without any specific treatment. In both cases edema was not due to allergic reaction, but rather due to higher then necessary volume of the product injected in the periorbital area, especially into the blepharal tissues. That was taken into account during the following sessions and the events have not occurred again. Overall the tolerability of the product was evaluated as very good both by participants and investigators. There were no allergic reactions observed during this trial.
Subjective evaluation of obtained results has shown positive effect in all treated volunteers (Figure 1). Complete absence of improvement was not observed in a single patient. Half of the cohort results were evaluated as good, excellent – in 12%, and moderate in 27% (Figure 1).
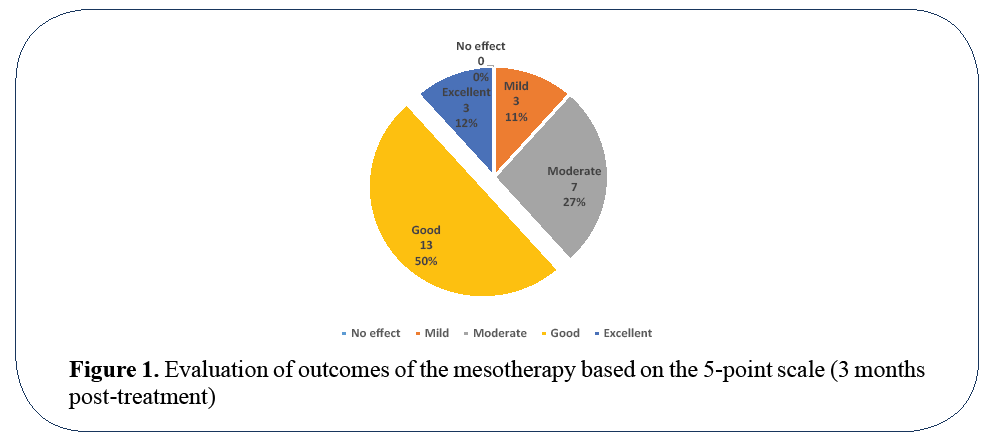
Figure 1: Evaluation of outcomes of the mesotherapy based on the 5-point scale (3 months post-treatment)
Evaluators have observed improvements in skin radiance and skin brightness in all volunteers. The parameters reflecting the skin brightness has shown gradual improvement since third week of the study. The spectrophotometric analysis has shown significant reduction of skin redness and pigmentations. Most dramatic changes in terms of decreased wrinkle height and maximum wrinkle depth were observed in periorbital (“crow’s feet”) and perioral areas. Total wrinkle height was decreased by 27.4% by the end of the treatment protocol, 25.1% one month post-treatment and 21.5%-3 months posttreatment. The maximum wrinkle depth was decrease by 20.9% by the end of the treatment at 12th week, by 17.8% - one month post-treatment, and 15.6% - 3 months post-treatment (Figure 2).
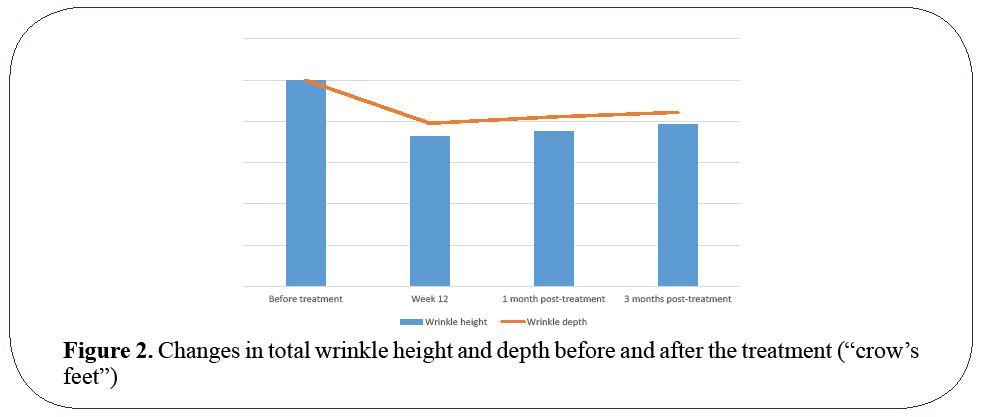
Figure 2: Changes in total wrinkle height and depth before and after the treatment (“crow’s feet”)
All tested individuals have demonstrated reduction of average skin roughness after the treatment with MF+ MO™ SPMCE. On the average significant improvements were observed on the third week of the trial. Prominent changes were also observed in the skin of the neck and chin. Neck area has reduced deep folds and improved microrelief of the skin.
Majority of patients had improvements in skin hydration and elasticity that were observed after first month of treatment. During the second and third months further improvements were less prominent, however the effects gained in the initial stages of treatment demonstrated high stability and sustained even 3 months after the last mesotherapy session (Figure 3).
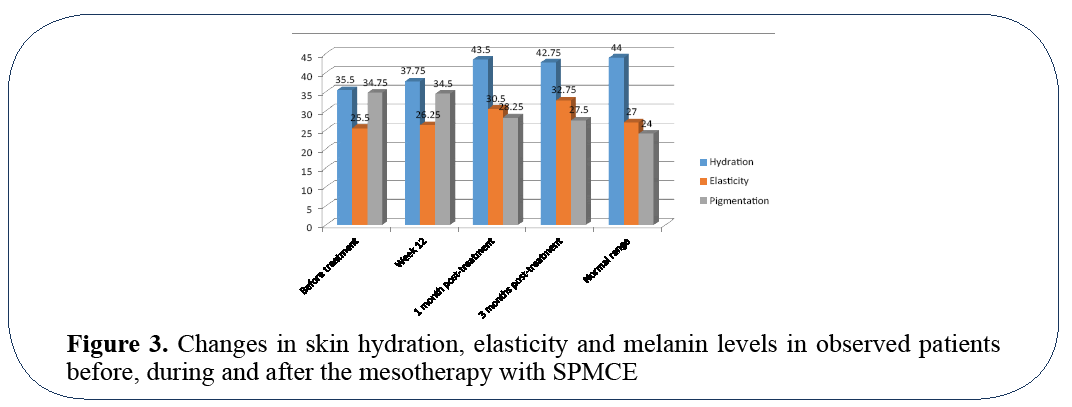
Figure 3: Changes in skin hydration, elasticity and melanin levels in observed patients before, during and after the mesotherapy with SPMCE
The main improvements of the cheek area included reduction of cheek ptosis, increased hydration and significantly reduced uneven pigmentations (Figure 4).
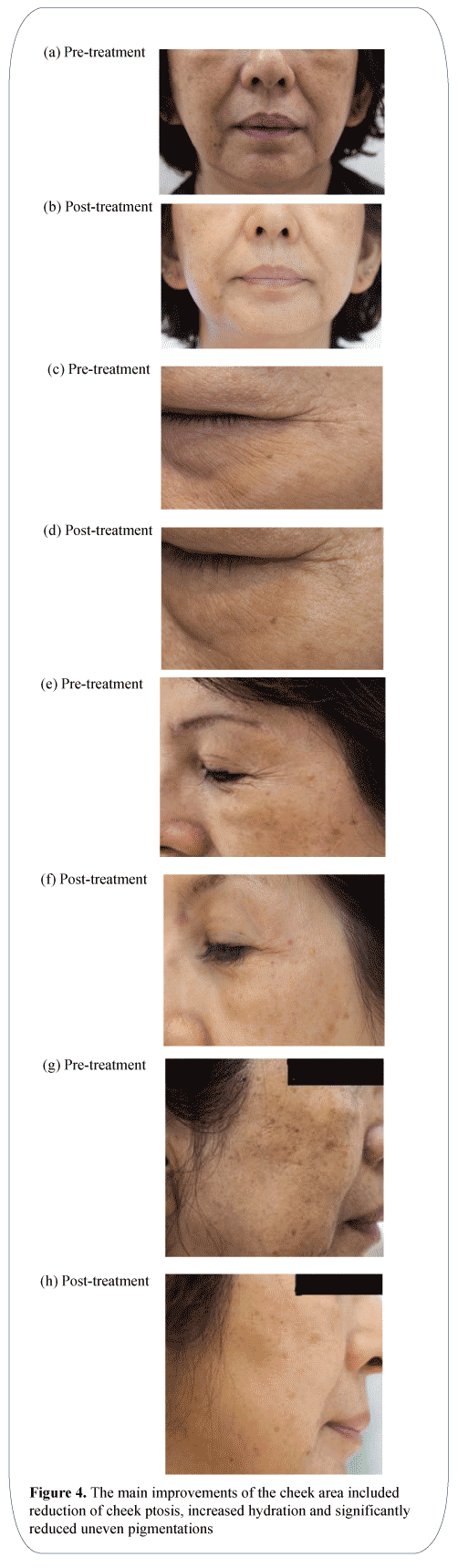
Figure 4: The main improvements of the cheek area included reduction of cheek ptosis, increased hydration and significantly reduced uneven pigmentations
Histoimmunostaining revealed slight increase of collagens types I and III – 67.5% to 71.6% and 64% to 72.5% respectively (p≥0.05), while the most significant difference after the treatment was observed in content of collagen type VII – 9.5% to 15.5% (pi0.05) (Figure 4). Picrosirius red staining observed under polarized light microscopy shown a statistically significant increase of newly synthesized collagen after the treatment (p¡0.05).
The biosynthesis of collagen type VII is known to progressively decrease with aging. The main reason for that is age-related degradation of keratinocytes and fibroblasts that are responsible for production of collagen type VII. Statistically significant increase of collagen type VII production provides a quantitative proof to the actual skin rejuvenation properties of the tested product (Figure 5).
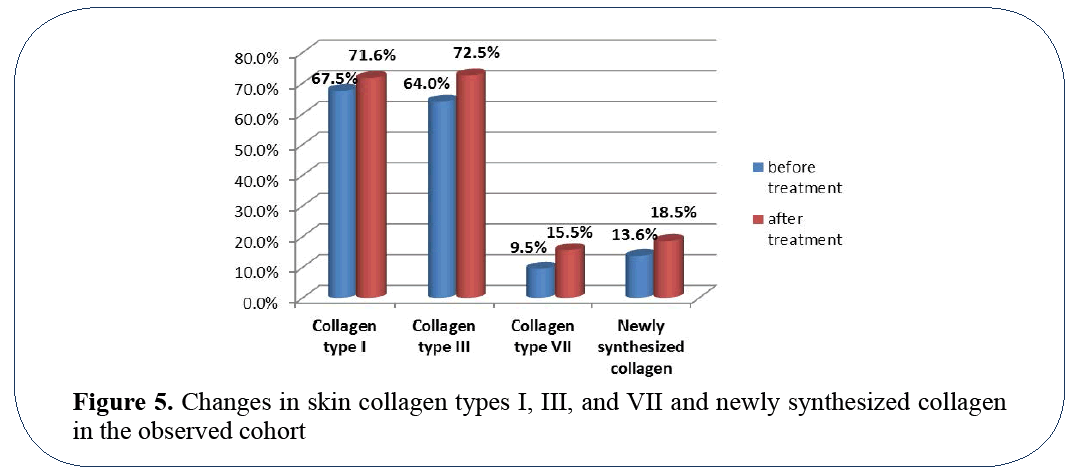
Figure 5: Changes in skin collagen types I, III, and VII and newly synthesized collagen in the observed cohort
Normally elastic fibers form 2-4% of the extracellular matrix and are responsible for skin elasticity. Thickening of elastic fibers, loss of its structure and organization form morphological background of solar elastosis and are some of the main characteristics of the aging skin. Elastic fibers consist of elastin, which is synthesized and its content evaluated in a form of tropoelastin. The observed changes of tropoelastin level after the treatment with MF+ MO™ SPMCE are reflected in Figure 5. The proposed mesotherapy formula is capable of providing a significant restoration of extracellular matrix and may dramatically improve skin elasticity.
As the main purpose of mesotherapy is revitalization and maintenance of youthful skin, a successful mesotherapy formula should be able to enhance cell activity and increase dermal cell turnover, as well as promote synthesis of collagen and elastin. The current study suggests that proposed product can achieve a physiological restoration of optimal environment in the treated tissues (Figure 6).
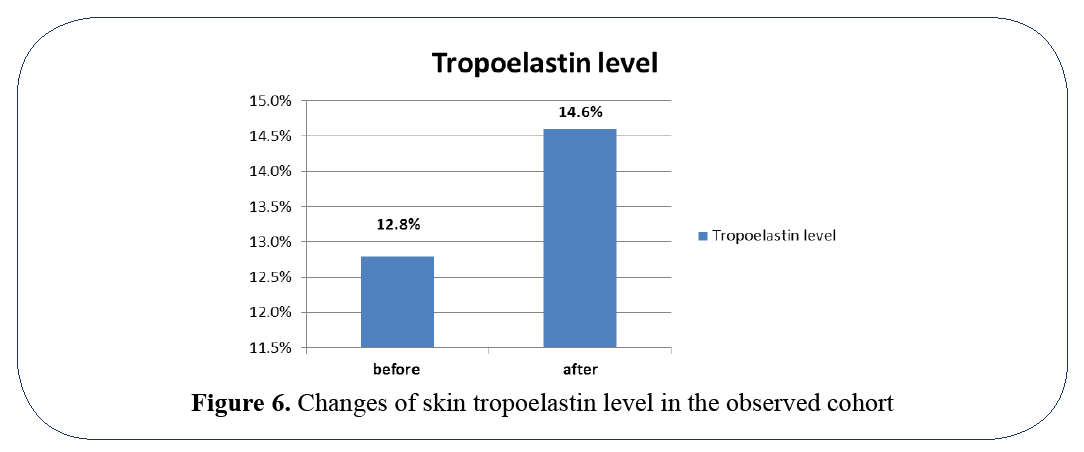
Figure 6: Changes of skin tropoelastin level in the observed cohort
Conclusion
Clinical and histochemical data obtained from the study confirms the anti-aging properties of the used product. Analysis of dermal elastic filaments has proven the reduction of skins roughness and determined the bio-revitalizing and rejuvenating effect of the used product. Study has demonstrated MF+ MO™ SPMCE capacity to promote biosynthesis of new collagen and elastic fibers. Even after the first month of treatment skin looked younger and more vibrant. In the end of the treatment protocol the improvements in skin hydration and dramatic reduction of senile lentigo and uneven pigmentation were noted.
One of the main benefits of the tested product are absolutely physiological mechanism of its action, not involving administration of the fillers or foreign bodies into the tissues, on one hand, and long-lasting natural biological revitalizing effect on the other hand. Absence of allergic reactions in the observed group supports safety of the MF+ MO™ SPMCE application in the aesthetic practice.
Taking into consideration the fact that concentration of peptides in the MF+ MO™ SPMCE is significantly higher compared to previous generation of cellular extracts of MF+ brand known as EUF or Nano extracts, as well as molecular weight of the peptides in the MF+ MO™ being slightly higher than in EUF/Nano extracts, we recommend to inject a smaller quantity of the product. The technique of intradermal injection and the volume of the injected product are essentially different from the ones using fillers or other known compositions containing vitamins or hyaluronic acid. Mesotherapy with MF+ MO™ SPMCE is the mesotherapy-as-it-is: “a little volume, a few times, and in the right place”.
Usually one vial is enough for the entire session of facial mesotherapy and the duration should not be more than ones per week. Tiny amounts of the product should be introduced proportionally to the areas of concern without over-pumping the tissues to prevent occurrence of edema. If some swelling is noted after repeated sessions we would suggest either further dilute the product or switch to EUF/Nano extracts with the similar content, but lesser concentration.
Overall, the obtained data allow concluding that application of MF+ MO™ SPMCE cellular extracts/peptides in mesotherapy can be considered as safe and effective method of facial rejuvenation.
References
- Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462-70.
- Peres PS, Terra VA, Guarnier FA, et al. Photoaging and chronological aging profile: Understanding oxidation of the skin. J Photochem Photobiol B. 2011;103:93-7.
- Sjerobabski MI, Poduje S. Photoaging. Coll Antropol 2008;32:177-80.
- West MD. The cellular and molecular biology of skin aging. Arch Dermatol. 1994;130:87-5.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-68.
- Situm M, Bulat V, Buljan M, et al. Senile lentigo – cosmetic or medical issue of the elderly population. Coll Antropol. 2010;34:85-8.
- Kligman LH. Photoaging: Manifestations, prevention, and treatment. Clin Geriatr Med. 1989;5:235-51.
- Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40-50.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-05.
- Kon A, Takeda H, Ito N, et al. Tissue-specific downregulation of type VII collagen gene (COL7A1) transcription in cultured epidermal keratinocytes by ultraviolet A radiation (UVA) and UVA-inducible cytokines, with special reference to cutaneous photoaging. J Dermatol Sci. 2005;1:29-35.
- Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93-105.
- Iorizzo M, De Padova MP, Tosti A. Biorejuvenation: theory and practice. Clin Dermatol. 2008;26:177-81.
- Toledo LS. Emerging techniques in aesthetic plastic surgery. Clin Plast Surg. 2009;36:177-80.
- Galadari H, Al Faresi F. Mesotherapy. Skinmed 2011;9:342-3.
- Pistor M. What is mesotherapy? Chir Dent Fr. 1976;46:59-60.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-80.
- Tosti A, De Padova MP. Atlas of mesotherapy in skin rejuvenation. London: Informa Healthcare; 2007.
- El-Domyati M, Attia S, Saleh F, et al. Effect of topical tretinoin on photoaged facial skin: a histometric, immunohistochemical and ultrastructural study. J Cosmet Dermatol. 2004;3:191-201.
- Rohrich RJ. Mesotherapy: what is it? Does it work? Plast Reconstr Surg. 2005;115:1425.
- Kon A, Takeda H, Ito N, et al. Tissue-specific downregulation of type VII collagen gene (COL7A1) transcription in cultured epidermal keratinocytes by ultraviolet A radiation (UVA) and UVA-inducible cytokines, with special reference to cutaneous photoaging. J Dermatol Sci. 2005;1:29-35.
- Mahoney MG, Brennan D, Starcher B, et al. Extracellular matrix in cutaneous aging: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp Dermatol. 2009;18:205-11.
- El-Domyati et al. Efficacy of mesotherapy in facial rejuvenation: a histological and immunohistochemical evaluation. Int J Dermatol. 2012;51: 913-19.
- Takiwaki H. Measurement of skin color: practical application and theoretical considerations. J Med Invest. 1998;44:121-26.
- Fullerton A, Fischer T, Lahti A, et al. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1996;35:1-10.
- Hoppe U, Sauermann G, Lunderstadt R. Quantitative analysis of the skin’s surface by means of digital signal processing. J Soc Cos Chem. 1985;36:105-23.
- Kautzky F, Dahm MW, Drosner M, et al. Direct profilometry of the skin: its reproducibility and variability. Journal of the European Academy of Dermatology and Venereology. 1995;5:15-23.
- Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996.
- Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97-104.
- Whittaker P, Kloner RA, Boughner DR, et al. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397-410.