Keywords
Essential oil; Prunus avium; in vitro fermentation; Sheep; Antioxidant activity
Introduction
Tunisia is characterized by four important breeds which are Barbarine, Fine Tail West, Black of Thibar and Sicilian-Sardinian with a total of 3736820 female units [1] and a number of breeders estimated at 274,000 which is more than twice the number of dairy cattle farmers in Tunisia [2]. For many years, antibiotics have been used as growth promoters for livestock [3]. In 2001 and according to the World Health Organization, this use was estimated at 50% of the antibiotics produced in the world. However, these substances appeared to have favoured the emergence of a large number of resistant bacterial strains and allergic reactions in consumers [3]. In 2006, the use of antibiotics to improve the growth and performance of animals was banned in the European Union. This has led to the reappearance of pathogens responsible for diseases and economic losses [4]. As a result, considerable efforts have been made to develop alternatives to antibiotic substitution. Among these alternatives, essential oils are receiving increasing attention as substitutes for natural antibiotics and also as additives for the manipulation of rumen fermentation [5]. Integrates our work that aims to enhance the essential oil of Prunus avium by adjusting the optimal dose for the increase of digestibility and improve ruminal fermentation parameters in sheep.
Materials and Methods
Animals material
The rumen content is taken from sheep at the TABARKA municipal slaughter house. It is transferred to the laboratory in a thermos preheated to 39°C.
Plant material
As part of this project, we will focus on the study of a new species that is Prunus avium, Sampling is carried out in spring in March and April, from two different regions. Tbeinia and Swiny et al. analyses were performed in triples within the laboratory of the Sylvo-Pastoral Institute of Tabarka.
Chemical and parietal composition
All samples were analysed for Dry Matter (DM), Ash, Organic Matter (OM), Crude Protein (CP), and fat content using the AOAC method [6]. The determination of the contents of ADF, NDF, ADL, crude cellulose and hemicelluloses are carried out according to the method [7].
Secondary metabolites and antioxidant activity
The determination of the total polyphenols, flavonoids and condensed tannins of the aqueous extracts are carried out respectively according to the methods of Singleton VL et al., Yi ZB et al., Sun B et al. [8-10]. The antioxidant activity of the aqueous extracts of different samples is evaluated by the DPPH test described by Ammar RB et al. [11]. The antioxidant activity of essential oils is evaluated according to the method described by Grzegorczyk I et al. [12].
In vitro fermentation
Monitoring the kinetics of gas production follows the addition of different doses of essential oil (0 μl; 10 μl; 30 μl; 50 μl; 100 μl) to rumen juice, a technique described by Spanghero M et al. [13] and based on the method of Menke KH et al. [14].
Statistical analysis
All data were subjected to statistical analysis by the variance according to the GLM procedure of the software [15] and compared by Duncan multiple rank tests. The model used was: Yij=μ+Ai+Eijk.
Where; Yij: dependent variable. μ: overall of Y; Ai: effect of the ith essential oil; Eijk: residual error
The characteristic parameters of the gas production kinetics are predicted according to the nonlinear regression by the use of the line procedure according to the model [15,16].
The equation of the model:
Y=a+b (1-exp-ct) where
Y: volume of gas produced after each incubation time (ml)
a: production of gas from the easily fermentable soluble fraction (ml)
b: production of gas from the insoluble, potentially fermentable fraction (ml)
c: gas production speed (h-1)
Results and Discussion
Chemical and parietal composition
Tables 1 and 2 present the chemical and parietal composition of Prunus avium. Indeed, the results show that the cherry tree is characterized by a low content of DM (29.66%) but a significant value in NDF and ADF respectively 44.4% and 32.75% of these results are in agreement with that presented by Ela hi MY et al.
DM%Ash%OM%Fat%CP%Prunus avium29.66 ± 17.03 ± 0.08592.97 ± 0.08547.933 ± 4.5413.85 ± 1.48
Table 1: Chemical composition of Prunus avium.
ADF%ADL%CF%NDF%HC%Prunus avium32.75 ± 137.24 ± 10.9511.36 ± 4.9144.44 ± 1.4211.69 ± 0.51
Table 2: Parietal composition of Prunus avium.
Secondary metabolites and antioxidant activity
The average content of total polyphenols, flavonoids and condensed tannins of Prunus avium illustrated in Table 3 shows a slight difference between what is found by Bastos C et al. [18]. In fact, the total polyphenol content is important 54.35 mgGAE/g DM, however, the contents of flavonoids and condensed tannins are low.
Polyphenols (mg GAE/g DM)Flavonoids (mg QE/g DM)Condensed tannins (mg CA/g DM)Prunus avium54.35 ± 3.696.79 ± 1.0627.82 ± 0.955
Table 3: Concentration of Prunus avium as secondary metabolites.
Table 4 shows the existence of a significant difference (p<0.01) between the samples tested for antioxidant activity. Indeed, the one with the lowest IC50 value is characterized by the most important antioxidant activity. it has been deduced that the aqueous extract has antioxidant activity (IC5=150.673 μg/ml) greater than that of essential oil (IC50=271.54 μg/ml) for ascorbic acid has the highest antioxidant activity (IC50=61.3 μg/ml). Our results are superior to those found by Bastos C et al. [18].
IC50 (µg/ml)Essential oil271.54a ± 1Aqueous extract150.673b ± 6.647Ascorbic acid61.3c ± 1p>F<0.0001 a: production of gas from the easily fermentable soluble fraction (ml)b: production of gas from the insoluble, potentially fermentable fraction (ml)c: gas production speed (h-1)
Table 4: Inhibition concentration of 50% of DPPH free radicals.
In vitro fermentation
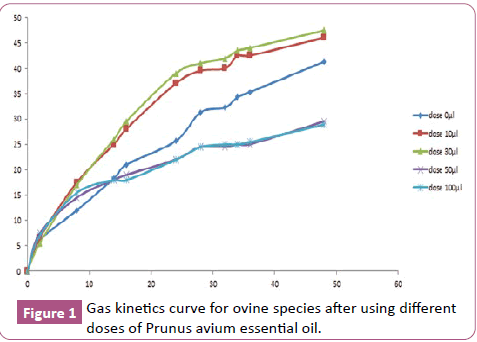
Figure 1 shows the gas productions recorded for oatmeal supplemented with EO at different concentrations (dose 10 μl, dose 30 μl, dose 50 μl, and dose 100 μl) while comparing the zero concentration. After 48 hours of incubation of Prunus avium HE decreases the production of gas with both concentrations 50 μl and 100 μl. While the use of low doses of 10 μl and 30 μl increases the production of gas. This can be explained by the fact that the EO of Prunus avium at a low dose of less than 30 μl increases the fermentation of oat hay and the increase in the EO concentration of Prunus avium induces a strong reduction in the production of gas. This decrease does not vary substantially between the two doses 50 μl and 100 μl which have the same pace of gas kinetics curve. This can be explained by the fact that the addition of EO at a dose greater than or equal to 50 μl greatly reduces the fermentation of oat hay and this reduction of gas can be linked to selective inhibition of the flora which degrades the Parietal fraction of oat hay. This suggests that this EO has a very strong antimicrobial activity on rumen microorganisms. However, the mechanism of action of EO is difficult to define because this extract consists of a very complex mixture of chemical molecules where each type of molecule has its own mechanism of action. So each dose belongs to the interval 10 μl and 30 μl are optimal doses and if we exceed 50 μl it can cause toxicity to the animal. The kinetic parameters of the in vitro fermentation of the different doses are shown in Table 5 deduced from the exponential model of Orsskov ER et al. [16]. They reveal, as for the biological model, that the highest value of the volume of gas is recorded for the dose 30 μl (47 ml), followed by 10 μl (46 ml), against the dose 50 μl displays the value the weaker (26.5 ml). The total gas production at 24 h of incubation expressed significant differences (p<0.01) between the different doses. Indeed, the averages are of the order of 46 ml, 47 ml, 26 ml and 29 ml respectively for the dose 10 μl, 30 μl, 50 μl and 10 μl. There is a significant difference between the different doses studied for parameter "c" (gas production rate (h-1) where the 10 μl and 30 μl doses have the highest speed.
Figure 1: Gas kinetics curve for ovine species after using different doses of Prunus avium essential oil.
abcG24GTControl2.723b ± 0.00558.83a ± 0.0110.02c25.66c ± 5.0341.33b ± 2.5110 µl1.64c ± 0.00550.43a ± 0.0050.05a37a ± 146a ± 130 µl0.3d ± 0.59830.64b ± 17.430.056a ± 0.02822c ± 147a ± 0,550 µl6.46a24.56b0.04b33b26.5c ± 5.50100 µl6.34a25.90b ± 0.0050.04b22c ± 1 29c ± 1P<0.00010.0002<0.0001<0.0001<0.0001a: production of gas from the easily fermentable soluble fraction (ml)b: production of gas from the insoluble, potentially fermentable fraction (ml)c: gas production speed (h-1)
Table 5: Characteristic parameters of the gas production of the different doses.
The digestibility of the OM, the Metabolizable Energy (ME) and the concentration of volatile fatty acids (total VFA) are grouped in Table 6. In fact, the doses studied show a significant difference (p<0.05) between the values of the digestibility of organic matter, the averages are of the order of 54.76% 51.2% 41.42% 41.42% respectively for 10 μl, 30 μl, 50 μl, and 100 μl. The metabolizable energy and the total VFA concentration were statistically different (p<0.01). The doses of EO 10 μl and 30 μl induce greater production of metabolizable energy and VFA. The use of HE in high concentrations strongly inhibits rumen flora, which decreases the digestibility of oat hay and the production of VFA [19]. This can be explained by the toxicity of EO to high levels. Depending on its chemical composition, low doses increase the fermentation and digestibility of oat fiber.
ME (Kcal/Kg DM) IVOMD%VFA mmol/syringeControl6.52b ± 0.244.68a ± 1.5070.55c ± 0.1210 µl8.06a ± 0.75754.76a ± 5.820.823b ± 0.02530 µl7.516a ± 0.6351.20b ± 5.005 0.73b50 µl6.02c ± 0.7541.426b± 5.830.466b± 0.025100 µl6.02c ± 0.75741.42b ± 5.8310.466b± 0.025p0.002150.000810.00104a: production of gas from the easily fermentable soluble fraction (ml)b: production of gas from the insoluble, potentially fermentable fraction (ml)c: gas production speed (h-1)
Table 6: Estimated digestibility parameters from the gas produced at 24 hours of incubation.
Conclusion
The analysis of the chemical composition and the prediction of the food value of Prunus avium has shown that this tree constitutes a natural resource in the northwestern areas of Tunisia which can contribute to the nutritional needs of small ruminants. Prunus avium essential oil represents a natural source of chemical molecules that at low doses can positively modify ruminal fermentation.
References
- European Patent Office (2017) Livestock and pasture office, Tunisia.
- Givlait (2005) Interprofessional group of red meats and milk, Survey on the structures of agricultural holdings.
- Corpet DE (1996) Microbiological hazards for humans of antimicrobial growth promoter use in animal production. J Vet Med 147: 851-862.
- Alloui MN (2011) Phytobiotics as an alternative to growth-promoting antibiotics in poultry feed. Livestock Rese Rural Develop 23: 133.
- Wallace RJ (2004) Antimicrobial properties of plant secondary metabolites. Proc Nutr Soc 63: 621-629.
- AOAC (1995) Official Methods of Analysis of the Association of Official Analytical Chemists, AOAC International, USA.
- Van Soest PJ, Maraus WC (1994) Method for the determination of cell wall constituents in forage, using detergents, and the relationship between this fraction and voluntary intake and digestibility. J Dairy 58: 704-705.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteaure agent method. Enzymol 299: 152-178.
- Yi ZB, Yu Y, Liang YZ, Zeng B (2007) In vitro antioxidant and antimicrobial activities of the extract of Pericarpium citri reticulatae of a new Citrus cultivar and its main flavonoids. LWT-Food Sci Tech 41: 597-603.
- Sun B, Richardo-da-Silivia JM, Spanger I (1998) Critical factors of vanillin assay for catechins and proanthocyanidins. J Agri Food Chem 46: 4267-4274.
- Ammar RB, Bhouri W, Sghaier MB, Boubaker J, Skandrani I, et al. (2009) Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae). Food Chem 116: 258-264.
- Grzegorczyk I, Matkowski A, Wysokinska H (2007) Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem 104: 536-541.
- Spanghero, M, Zanfi C, Fabbro E, Scicutella N, Camellini C (2008) Effects of a blend of essential oils on some end products of in vitro rumen fermentation. Ani Feed Sci Tech 145: 364-374.
- Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28: 7-55.
- SAS User’s Guide (1989) Version 6.10 for Windows, SAS Inst. Inc., Cary, NC.
- Orsskov ER, Macdonald I (1979) The estimations of protein degradability in the rumen from incubation measurements weighted according to rate passage. J Agri Sci Cambridge 92: 499-502.
- Elahi MY, Kargar H, Dindarlou MS, Kholif AE, Elghandou MM, et al. (2017) The chemical composition and in vitro digestibility evaluation of almond tree leaves versus hulls and green versus dry leaves as feed for ruminants. Agroforestry Systems 91: 773-780.
- Bastos C, Barros L, Dueñas M, Calhelha RC, Queiroz MJR, et al. (2015) Chemical characterization and bioactive properties of Prunus avium L.: the widely studied fruits and the unexplored stems. Food Chem 173: 1045-1053.
- Chaney AL, Marbach EP (1962) Modified reagents for determination of urea and ammonia. Clin Chem 8: 130-132.