Ekaterina S Snigirevskaya*, Alexej V Moshkov and Yan Yu Komissarchik
Department of Cell Morphology, Institute of Cytology of the Russian Academy of Sciences, St. Petersburg, Russia
Corresponding Author:
Ekaterina S. Snigirevskaya
Department of Cell Morphology, Institute
of Cytology of the Russian Academy
of Sciences, St. Petersburg, Russia
Tel: 7(812)2976302
E-mail: snigir@incras.ru
Received Date: September 18, 2017 Accepted Date: October 12, 2017 Published Date: October 17, 2017
Citation: Snigirevskaya ES, Moshkov AV, Komissarchik YY (2017) Ultrastructural Study and X-Ray Microanalysis of Apoptotic Lymphoma Cells U-937. Biochem Mol Biol J Vol. 3: No. 3:12. doi: 10.21767/2471-8084.100040
Description
We studied the ultrastructure and ionic cell contents of human histiocytic lymphoma U-937 cells induced for apoptosis by two external agents, hypertonic shock and etoposide. Despite different mechanisms of action, the deterioration of cellular functions triggered by these agents followed similar patterns. The most dramatic changes–condensation of the chromatin and formation of “crescent-shaped nuclei”, nucleus fragmentation, disappearance of nucleoli, and appearance of aggregated particles in the interchromatin nuclear space were observed in the nucleus. The aggregated particles observed in the interchromatin nuclear space, were lacking limiting membrane and presumably derived from the aggregates of interchromatin granules (IGs), so called speckles. The speckles, were shown previously to be highly dynamic nuclear structures associated with: m-RNA-splicing, transcription, and processing of pre-mRNA [1]. We attribute the described here aggregates to the “HERDS” (heterogeneous ectopic RNP-derived structures) type described by Biggiogera and co-authors [2,3]. In contrast to other cytoplasmic aggregates such as aggresomes, processing bodies (Pbs) or stress bodies (SBs) HERDS were observed both in the nucleus, and in cytoplasm [4,5]. They emerge in response to impairment of transcription during apoptosis. In case of cellular physiological modifications, e.g. under spermiogenesis or animal hibernation, this process can be reversible [2,3]. Ultrastructural analysis and immune electron microscopy of the aggregates observed in U-937 cell line allowed identification of three types of nuclear particles dominating in these aggregates: interchromatin granules (IGs) of 10 x 40 nm, proteasomes (PRs) of 10-15 × 30 nm, and long perichromatin fibers (PFs) [4]. Both IGs and PRs were rod-like shaped particles of variable size. They demonstrated different immune properties: IGs recognized ABs against the splicing factor SC35 (Figure 1a), meanwhile PRs reacted with antibodies (ABs) against proteins of the proteasome protein complex (Figure 1b). Ultrastructurally PFs observed in this study essentially differed from the ones described earlier [3-6].
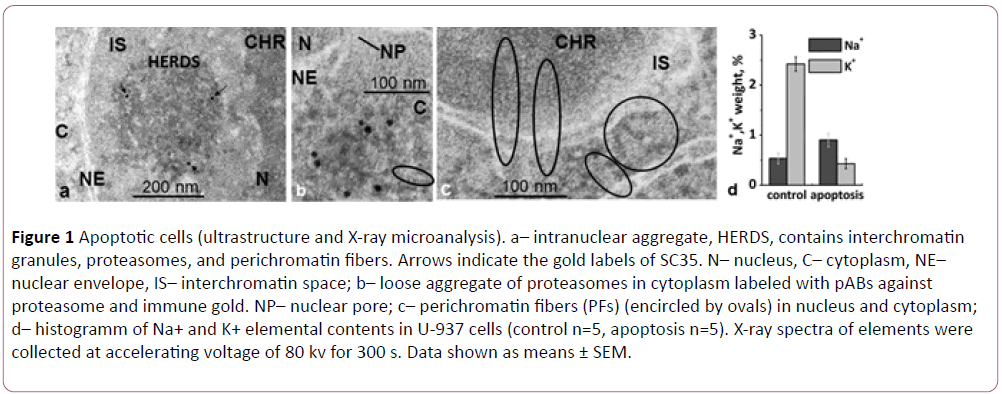
Figure 1: Apoptotic cells (ultrastructure and X-ray microanalysis). a– intranuclear aggregate, HERDS, contains interchromatin granules, proteasomes, and perichromatin fibers. Arrows indicate the gold labels of SC35. N– nucleus, C– cytoplasm, NE– nuclear envelope, IS– interchromatin space; b– loose aggregate of proteasomes in cytoplasm labeled with pABs against proteasome and immune gold. NP– nuclear pore; c– perichromatin fibers (PFs) (encircled by ovals) in nucleus and cytoplasm; d– histogramm of Na+ and K+ elemental contents in U-937 cells (control n=5, apoptosis n=5). X-ray spectra of elements were collected at accelerating voltage of 80 kv for 300 s. Data shown as means ± SEM.
PFs on sections contrasted with uranyl-acetate were seen as elongated electron dense particles 12-13 nm thick with a periodic structure with a period of 10 nm (Figure 1c) [7]. PFs were formed by 4 nm thick helical threads. In our experiments application of uranyl-acetate essentially increased electron density of PFs, suggesting presence RNA- and RNP-containing structures which are known to stain by uranium salts [8]. We demonstrated translocation of PFs from the nucleus to cytoplasm via nuclear pores (Figure 1c). Previously it was hypothesized that PRs were transferred to cytoplasm in a same way [9]. Therefore, we were the first to directly demonstrate that the intranuclear/intracytoplasmic aggregates named HERDS are composed of three morphologically distinct types of particles, IGs, PRs, and PFs. We followed the dynamics of the aggregate formation, transport of the aggregate components from the nucleus to cytoplasm, and their release into the extracellular space with the help of exosomes during the terminal stage of the apoptosis.
By application of X-ray microanalysis, we were able to detect changes in the intracellular contents of Na+ and K+ during apoptosis induced by osmotic shock, at the single cell level. An increased ratio of intracellular Na+/K+ was detected for majority of the cells entering apoptosis (Figure 1d). These results are consistent with our previous study [5].
Acknowledgements
The authors thank Dr. Yuliya Y. Sokolova for editing the final version of the manuscript.
References
- Spector DL, Lamond AI (2011) Nuclear speckles. Cold Spring Harb Perspect Biol 3: a000646.
- Biggiogera M, Bottone MG, Pellicciari C (1997) Nuclear ribonucleoprotein-containing structures undergo severe rearrangement during spontaneous thymocyte apoptosis. A morphological study by electron microscopy. Histochem Cell Biol 107: 331-336.
- Biggiogera M, Bottone MG, Scovassi AI, Soldani C, Vecchio L, et al. (2004) Rearrangement of nuclear ribonucleoprotein (RNP)-containing structures during apoptosis and transcriptional arrest. Biol Cell 96: 603-615.
- Snigirevskaya ES, Komissarchik YY (2017) Ultrastructural analysis of human leukemia U-937 cells after apoptosis induction: Locaization of proteasomes and perichromatin fibers. Acta Histochem 119: 471-480.
- Snigirevskaya ES, Moshkov AV, Yurinskaya VE, Vereninov AA, Komissarchik YY (2015) Ultrastructural and X-ray analysis of U-937 cells in hipertenzion induced apoptosis. Cell Tiss Biol 9: 96-109.
- Kotaja N, Sassone-Corsi P (2007) The chromatoid body: A germ-cell-specific RNAprocessing centre. Nat Rev Mol Cell Biol 8: 85-90.
- Fakan S (1994) Perichromatin fibrils are in situ forms of nascent transcripts. Trends Cell Biol 4: 86-90.
- Mironov AA, Komissarchik YY, Mironov VA (1994) Methods of Electron Microscopy in Biology and Medicine. SPb, Nauka, pp: 496.
- Enenkel C (2017) Proteasome dynamics: Will the territory of proteasomes be claimed by mitochondrial proteases Under Stress Conditions? Biochem Mol Biol J 2: 1-3.


