Research Article - (2015) Volume 1, Issue 1
Ganesh Chandra Jagetia1* and Shaival K Rao2
1Department of Zoology, Mizoram University, Aizawl-796 004, India
2Department of Pharmacognosy, C. U. Shah College of Pharmacy & Research, Wadhwan, Gujarat, India
Corresponding Author:
Dr. Ganesh Chandra Jagetia
Professor and Head, Department of Zoology, Mizoram University
Aizawl-796 004, Gujarat, India
Tel:91-389-2330724
E-mail: gc.jagetia@gmail.com
Received Date: August 17, 2015 Accepted Date: November 20, 2015 Published Date: November 26, 2015
Environmental science is moving through a dreaded and drastic crisis. Tinospora cordifolia or giloe is used in Ayurvedic system of medicine for the treatment of many diseases. Our earlier studies have shown that giloe exerts cytotoxic effect in vitro and in vivo, which stimulated us to understand the mechanism of cell death is HeLa cells receving various concentrations of giloe. Therefore, induction of molecular DNA damage by different concentrations of dichloromethane extract of giloe, Tinospora cordifolia (TCE) was studied in HeLa cells by alkaline comet assay and the DNA damage induced by TCE has been expressed as olive tail moment (OTM). Incubation of HeLa cells with TCE for 4 h caused greater DNA damage as indicated by increased OTM when compared with 2 h treatment and therefore 4 h exposure time was considered as an optimum duration for TCE treatment. Exposure of HeLa cells to 0, 1, 2, 4, 6 or 8 μg/ml TCE resulted in a concentration dependent elevation in the DNA damage and the lowest concentration of 1 μg/ ml TCE increased the baseline DNA damage approximately by 10 folds, whereas 8 μg/ml TCE, the highest concentration tested caused an approximate 68 folds elevation in the DNA damage, when compared with the untreated control.
Introduction
Phytoceuticals have been used in traditional medicinal systems for healthcare in India and China since the advent of thier civlizations. The phytoceuticals and other natural products continue to play a cucial role role in human healthcare in developning and developed countries.World Health Organization estimates indicate that approximately 80% of humans worldwide mainly use traditional medicines for their primary healthcare [1]. The natural products and botanicals are easily accesible, and econimacally less expensive than the synthetic compounds. They have also been belived to known toxic as their use has been since the advent of human history [2,3]. This has been the prime reason of their poplarity among human population. Despite the fact that plants have a long history of use in the treatment of cancer, many, if not all, of the claims for the efficacy of such treatment are viewed with skepticism because cancer, as a specific disease entity, is poorly defined in terms of folklore and traditional medicinal systems. It is also well known that some of the best known modern cancer treatment drugs including vinca alkaloids, vinblastine, vincristine, taxol, phodophyllotoxins, and topotocan etc. have been originally isolated from various plant species and had a history of long traditional use [3]. Phytoceuticals offer as an alternative to the synthetic compounds and a great promise in the treatment of cancer as they are practically non-toxic or have lesser toxic implications than the synthetic molecules [4]. The crude extracts from plants like Alstonia scholaris, and Aegle marmelos have been reported to show a marked tumor inhibitory activity in vitro and in vivo [5-7]. Similarly, the extract of Aphanamixis polystachya has been reported to show marked antitumor activity and enhance the effect of radiation in tumor bearing mice earlier [8,9].
Herbal products are used in different forms for human healthcare. Herbs are used in the form of crude extracts or mixer of crude extracts as practiced by the traditional healers or alternatively, the active molecules from various botanicals are isolated and used for the treatment of various human ailments including cancer, which is commonly practiced by the modern system of medicine [4,10]. Tinospora cordifolia Miers, belonging to family Menispermaceae, is commonly known as guduchi or giloe is a non-toxic herbal preparation, which has been widely used in the Ayurvedic system of medicine for its general tonic, antiinflammatory, antibacterial, antiviral, antimalarial, antileprotic, hypoglycemic, immunomodulatory and aphrodisiac properties [11-17]. Different extracts of giloe were found to protect against carbon tetrachloride-induced hepatotoxicity in rats [18]. It has been found to alleviate cisplatin-induced nephrotoxicity in vivo [19,20]. Giloe has been also reported to be antimutagenic and active against HIV virus [21,22]. It has been used in the treatment of human throat cancer clinically [23]. Giloe has been found to be non-toxic pharmacophore and earlier studies from this laboratory have reported that the dichloromethane extract of giloe is non-toxic up to a dose of 1.2 g/kg in mice [24,25]. Similarly, methanol extract of giloe has also been found to be non-toxic up to a dose of 3.5 g in mice and rats [26]. The alcoholic extract of giloe has been reported to attenuate the cyclophosphamide-induced urotoxicity in vivo [27]. The use of different giloe extracts have been reported to kill cervical cancer cells in a concentration dependent manner [28,29]. Giloe has been reported to increase the cell killing effect of radiation in vivo and in vitro earlier [25,30]. A recent study has shown that dichloromethane extract of giloe increased the effect of radiation by elevating the radiation-induced DNA damage as assessed by comet assay [31]. The crude extract of another species of giloe, Tinosposa crispa has been also found to exert cytotoxic effect on HeLa, MCF-7, MDAMB-231 and 3T3 cells in vitro [32].
The prediction of response to therapy is of utmost importance for the treatment of cancer and the early detection of response to therapy allows physicians to devise proper regimens for better tumor cure. Several methods have been used to predict response of tumor including imaging and gene expression [33,34]. However, the most efficient method will be that which can detect the DNA damage in individual cancer cells. It also well recognized that the most active and efficient anticancer drugs are those that damage cellular DNA and subsequently reduce the clonogenic potential of the cells. Therefore, it is seems prudent to estimate the DNAdamaging effect of antineoplastic agents on normal and cancer cells [35]. The comet assay is a simple technique that allows the determination of the amount of DNA damage (both single and double strand breaks and conformational changes) in a single cell exposed to DNA damaging agents after removal of the most of the non-DNA material and application of a weak electric current to the remaining DNA embedded in an agarose gel matrix [36]. Usually, the whole cells are impregnated into an agarose gel, lysed and treated in situ with alkali to render the DNA single-stranded before subjecting the gel to electric current. The application of appropriate electrical field causes the genomic DNA to migrate out of the nucleus into the agarose. Subsequently the DNA is stained with the intercalating fluorescent dye ethidium bromide, which allows visualization of the DNA under a microscope equipped with fluorescence attachment. Under the fluorescence microscope, the combination of the DNA that has remained in the nucleus (head) and the DNA that has migrated (tail) out of the nucleus makes individual cells look like a comet and hence the name comet assay [36]. The DNA damage is quantitatively estimated under the microscope by measuring the length and intensity of the comet in relation to the signal of the nonmigrating nuclear DNA in comparison with standards [36,37].
Giloe extract has been reported to induce micronuclei and chromosome aberrations in a concentration dependent manner in HeLa cells in our earlier studies [28,38]. However, the mechanism of action of giloe extract on cell killing remains to be elucidated. The alkaline comet assay can detect double and single strand breaks, alkali-labile sites that are expressed as single strand breaks and single strand breaks associated with incomplete excision repair [36]. Therefore, an attempt has been made to elucidate the molecular DNA damage caused by giloe extract in HeLa cells by alkaline comet assay and correlate it with the cell survival.
Materials and Methods
Drugs and chemicals
The methylene chloride stem extract of Giloe, Tinospora cordifolia (TCE) was provided by Krüger Pharmaceuticals (Bombay, India). Minimum Essential Medium (MEM), L-glutamine, gentamycin sulfate, fetal calf serum, normal and low melting point agarose (LMA, Cat No. A-4718), ethylenediamine tertra acetic acid (EDTA), Triton-X, and Trizma base, were supplied by Sigma Aldrich Chemical Co., Bangalore, India. Dimethylsulfoxide (DMSO) and other routine chemicals were obtained from the Ranbaxy fine Chemicals, Mumbai, India.
Preparation of drug solution
TCE solution was prepared by dissolving 5 mg/ml of TCE in dimethyl sulfoxide (DMSO) and diluted in MEM in such a way so as to obtain concentrations of 1, 2, 4, 6 or 8 μg/ml, respectively immediately before its use. The concentration of DMSO never exceeded 0.2%. All drug solutions were prepared afresh immediately before use.
Cell line and culture
HeLa S3 cells were obtained from the National Centre for Cell Science, Pune, India, and were used throughout the study. The doubling time of HeLa S3 cells is 20 ± 2 h. The cells were routinely grown in 25 cm2 culture flasks (Cellstar, Greiner, Germany) with loosened caps containing Eagle's minimum essential medium (MEM) supplemented with 10% fetal calf serum, 1% L-glutamine and 50 μg/ml gentamicin sulfate at 37°C in an atmosphere of 5% CO2 in humidified air in a CO2 incubator (NuAir, Plymouth, USA).
Experimental Design
Usually, 5 × 105 exponentially growing HeLa cells were inoculated into multiple culture flasks (Techno Plastic Products, Trasadingën, Switzerland). The cells were allowed to reach to plateau phase. The cell cultures or cells impregnated in agarose (details are given in comet assay section) were divided into the following groups according to the treatment:
MEM group: The cells of this group were treated with 2 μl/ml of DMSO.
TCE group: This group of cells was treated with 1, 2, 4, 6 or 8 μg/ ml of TCE.
Selection of optimum time
A separate experiment was conducted to determine the optimum treatment duration of TCE treatment, where the HeLa cells embedded in agarose from either group were treated with DMSO or 1, 2, 4, 6 or 8 μg/ml of TCE for two or four hours to assess the DNA damage.
DNA repair kinetics
A separate experiment was carried out to assess the DNA damage induced by various doses of TCE at 0, 0.5, 1, 2, 4, 6, 10, 12, 16, 18 or 24 h post-TCE treatment. The experimental design was essentially similar to that described above except that the HeLa cells were exposed to TCE for 4 h.
Clonogenic assay
Clonogenic assay was performed to correlate the cell survival with DNA damage [39]. Briefly, 200 HeLa cells were inoculated into several individual culture dishes (Cellstar, Greiner, Germany) containing 5 ml drug free medium in triplicate for each TCE concentration. The cells were exposed to 0, 1, 2, 4, 6 or 8 μg/ml of TCE for 4 h and allowed to grow for 11 post-treatment days. The colonies obtained after 11 days were stained with 1% crystal violet in methanol and clusters containing 50 or more cells were scored as a colony. The plating efficiency of cells was determined and the surviving fraction was fitted on to a linear quadratic model SF=exp–(αD+βD2).
Alkaline comet assay
The DNA damage for both experiments was assessed by alkaline comet assay as described earlier [36,40]. The comet assay was carried out by embedding the cells in agarose, followed by lysis in alkaline buffer and subjecting the gel to an electric current finally. The application of electric current pulled the charged DNA from the nucleus leading to the migration of the relaxed and broken DNA fragments away from the nucleus than the intact DNA, which stays in the nucleus. The resulting images looked like stellar comets, and the fluorescence intensity from each comet was measured to determine the extent of DNA damage [36,41]. The DNA damage in HeLa cells was determined by impregnating cells in agarose on microscope slides and treating them with DMSO (MEM group) or TCE either for 2 or 4 h (optimum time study) or 4 h (DNA repair kinetics). Briefly, the one side frosted slides were covered with 100 μl of 0.6% low melting agarose (prepared in Ca2+ and Mg2+ free PBS at 37°C). The agarose coated slides were covered with a cover slip and placed on to ice to allow solidification of the agarose, thereafter the cover slips were removed. The aliquots of 1 ml containing 1 × 105 harvested HeLa cells in culture medium were centrifuged at 1500 rpm for 5 min. The pelleted cells were resuspended in 80 μl of 0.6% low melting agarose that was layered on to the first layer and allowed to solidify under a cover slip on ice. For repair slides, equal volumes of cell suspension (in MEM) and 1.2% LMA (dissolved in MEM) were mixed, layered and allowed to solidify under a fresh cover slip on ice. All the steps were conducted under diffused light to prevent additional DNA damage.
The frosted slides impregnated with cells, were kept for 2 h in the cold lysis buffer containing 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Trizma base (pH 10) and 1% Triton X-100 (added afresh) to solubilize cellular proteins leaving DNA as nucleoids. After cell lysis, the slides were drained of lysis buffer and placed into a horizontal gel electrophoresis tank filled with fresh electrophoresis buffer containing 300 mM NaOH, 1 mM Na2EDTA (pH 13) to a level of ~0.25 cm above the slides. The slides were left in the buffer for 20 min so as to allow DNA unwinding. The electrophoresis was carried out for 20 min at 1.25 V cm-1 and 300 mA in cold. The slides were drained and flooded slowly with three changes of neutralization buffer (0.4 M Trizma base, pH 7.5) for 5 min each. The cells were subsequently stained with 50 μl of ethidium bromide (2 mg/ml) and covered with a coverslip for immediate analysis under a fluorescence microscope.
The DNA stained with ethidium bromide on each slide was visualized at 40 × magnification using fluorescence microscope as “comets” with a distinct fluorescent head and a tail (Figure 1). The comet images were acquired using an epifluorescence microscope (Olympus BX51, Olympus Microscopes, Tokyo, Japan) equipped with a 515-535 nm excitation filter, a 590 nm barrier filter, and a CCD (charged coulped device) camera (CoolSNAP-Procf Digital Color Camera Kit Ver 4.1, Media Cybergenetics, Silver Spring, Maryland, USA). Usually data from a total of 100 cells per slide were collected to give a representative result for the cell population [42]. The comet images thus captured were analysed using Komet Software (Version 5.5, Kinetic Imaging Ltd, Bromborough, UK). The mean olive tail moment (OTM) was selected as the parameter that best reflects DNA damage and it was calulated as Tail DNA% x Tail Moment Length. The tail moment length was measured from the center of the head to the center of the tail. OTM was measured from three independent experiments, each containing quintuplicate measures and presented as Mean ± SEM (Standard error of the mean).
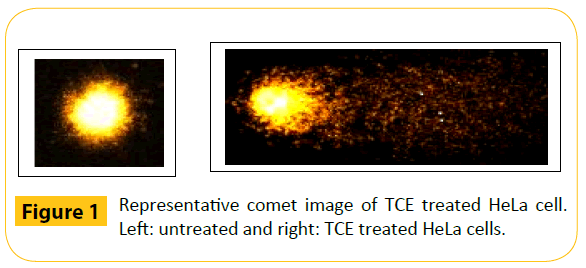
Figure 1: Representative comet image of TCE treated HeLa cell. Left: untreated and right: TCE treated HeLa cells.
Statistical analysis
The statistical analyses were performed using GraphPad Prism 5 Statistical Software (GraphPad Software, San Diego, CA, USA). The significance among all groups was determined by one-way ANOVA and Bonferroni’s post-hoc test was applied for multiple comparisons. The experiments were repeated for confirmation of the results. The results are the average of five individual experiments. The test of homogeneitywas applied to find out variation among each experiment. The data of each experiment did not differ significantly from one another and hence, all the data were combined and means calculated. A p value of <0.05 was considered statistically significant.
Results
The results are expressed as mean Olive Tail Moment (OTM) ± SEM in Tables 1 and 2 and cell survival in Figures 2-4.
| Concentration (µg/ml) | Olive Tail Moment (Mean ± SEM) | ||
|---|---|---|---|
| Incubation time | |||
| 2 h | 4 h | ||
| DMSO | 0.761 ± 0.061 | 0.84 ± 0.045 | |
| TCE (µg/ml) | 1 | 5.24 ± 0.052 | 8.61 ± 0.024 |
| 2 | 8.64 ± 0.041 | 11.55 ± 0.061 | |
| 4 | 13.51 ± 0.082 | 17.57 ± 0.044 | |
| 6 | 25.11 ± 0.04 | 29.39 ± 0.031 | |
| 8 | 43.23 ± 0.074 | 58.92 ± 0.064 | |
Table 1: Effect of different treatment times on the DNA damage measured as Olive tail moment (OTM) in HeLa cells exposed to various concentrations of giloe extract (TCE).
| Treatment time (h) |
Olive Tail Moment (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|
| DMSO | TCE (µg/ml) | |||||
| 1 | 2 | 4 | 6 | 8 | ||
| 0 | 0.86 ± 0.051 | 8.63 ± 0.023a | 11.58 ± 0.061a | 17.64 ± 0.044a | 28.91 ± 0.031a | 59.02 ± 0.064a |
| 0.25 | 0.88 ± 0.041 | 8.69 ± 0.024a | 11.81 ± 0.047a | 18.06 ± 0.033a§ | 29.22 ± 0.051a | 60.31 ± 0.066a* |
| 0.5 | 0.91 ± 0.056 | 9.42 ± 0.052a* | 12.27 ± 0.025a* | 19.37 ± 0.035a* | 31.38 ± 0.054a* | 66.44 ± 0.062a* |
| 1 | 0.92 ± 0.052 | 9.66 ± 0.0524a* | 13.37 ± 0.047a* | 20.22 ± 0.036a* | 33.55 ± 0.053a* | 69.12 ± 0.063a* |
| 2 | 0.92 ± 0.041 | 10.33 ± 0.054a* | 14.40 ± 0.048a* | 21.47 ± 0.047a* | 35.14 ± 0.051a* | 73.30 ± 0.074a* |
| 4 | 0.92 ± 0.035 | 12.45 ± 0.025a* | 16.55 ± 0.046a* | 23.28 ± 0.052a* | 38.20 ± 0.051a* | 75.92 ± 0.064a* |
| 6 | 0.92 ± 0.035 | 12.54 ± 0.056a* | 17.24 ± 0.051a* | 25.24 ± 0.042a* | 39.72 ± 0.057a* | 81.32 ± 0.062a* |
| 10 | 0.93 ± 0.042 | 11.98 ± 0.054a* | 17.56 ± 0.052a* | 25.81 ± 0.045a* | 40.38 ± 0.048a* | 81.64 ± 0.063a* |
| 12 | 0.90 ± 0.035 | 11.82 ± 0.054a* | 17.21 ± 0.052a* | 24.73 ± 0.035a* | 41.12 ± 0.049a* | 81.02 ± 0.061a* |
| 16 | 0.89 ± 0.056 | 10.8 ± 0.056a* | 17.13 ± 0.045a* | 24.12 ± 0.025a* | 41.34 ± 0.047a* | 80.22 ± 0.061a* |
| 18 | 0.89 ± 0.054 | 10.21 ± 0.047a* | 16.57 ± 0.048a* | 23.91 ± 0.027a* | 40.57 ± 0.048a* | 79.13 ± 0.059a* |
| 24 | 0.88 ± 0.013 | 10.07 ± 0.054a* | 16.02 ± 0.049a* | 22.57 ± 0.034a* | 39.67 ± 0.043a* | 78.04 ± 0.057a* |
a=p<0.001 (Comparison between TCE concentrations)
*=p<0.001; ♣=p<0.01 (when compared with 0 h)
No symbols=non significant
Table 2: Alteration in DNA damage in HeLa cells exposed to various concentrations of giloe extract (TCE) at different post-treatment times.
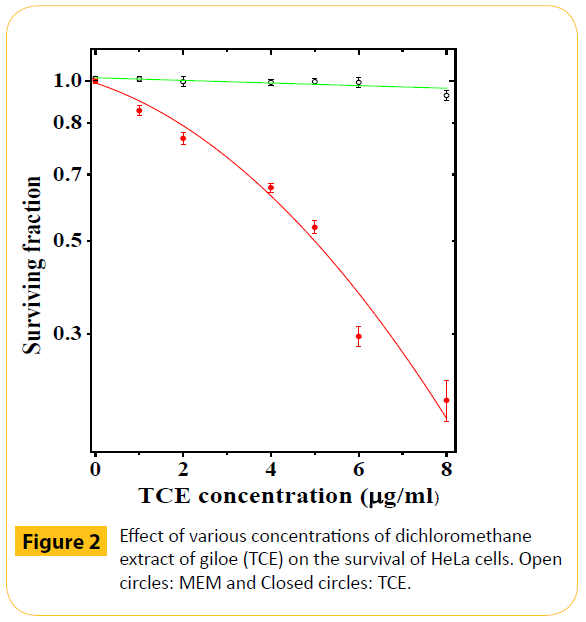
Figure 2: Effect of various concentrations of dichloromethane extract of giloe (TCE) on the survival of HeLa cells. Open circles: MEM and Closed circles: TCE.
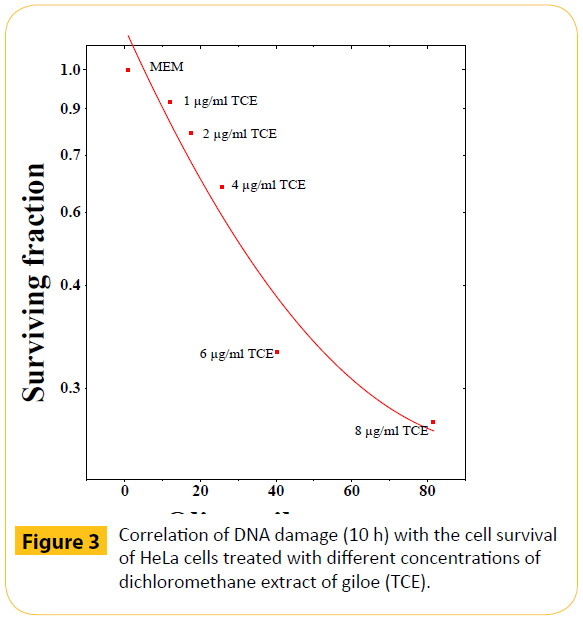
Figure 3: Correlation of DNA damage (10 h) with the cell survival of HeLa cells treated with different concentrations of dichloromethane extract of giloe (TCE).
Figure 4: Fourier Transform Infrared (FTIR) spectrum of berberine isolated from dichloromethane extract of giloe (TCE).
Selection of optimum treatment duration
Treatment of HeLa cells with DMSO either for 2 or 4 h did not induce baseline DNA damage significantly. However, treatment of HeLa cells with different concentrations of TCE increased DNA damage significantly (p<0.0001) at 2 h post-TCE treatment as indicated by increased OTM. Increase in TCE treatment duration of HeLa cells up to 4 h increased the DNA damage further when compared with 2 h (Table 1). Since a greater DNA damage was observed for 4 h treatment, further studies were conducted using this time of TCE exposure.
DNA repair kinetics
DMSO treatment did not alter the baseline DNA damage in HeLa cells significantly at different post-treatment times (Table 2). HeLa cells treated with a lowest concentration of 1 μg/ml TCE accentuated the baseline DNA damage approximately by 10 folds, which was significantly higher when compared with DMSO treatment alone at all the post-treatment times (Table 2). A further increase in TCE concentration resulted in a concentration dependent elevation in DNA damage and the maximum rise in DNA damage was observed for 8 μg/ml TCE treatment. The DNA damage was significantly elevated in HeLa cells with each concentration of TCE when comparisons were made amongst various concentrations of TCE (p<0.001).
Evaluation of DNA damage after treatment with various concentrations of TCE showed a continuous elevation in DNA damage up to 6 h, thereafter a significant decline was observed for 1 μg/ml indicating repair of damaged DNA. The increase in TCE concentration up to 2–8 μg/ml resulted in a greatest elevation in the DNA damage at 10 h, except for 6 μg/ml TCE, where the DNA damage was maximum at 16 h post-TCE treatment. Despite this increase the differences between 10 and 16 h post treatment were statistically non-significant. Thereafter, a non-significant decline in DNA damage was seen up to 24 h post-treatment.
Clonogenic assay
The reproductive integrity of HeLa cells remained unaffected by DMSO treatment as evidenced by non-significant changes in the survival of HeLa cells (Figure 2). Use of various concentrations of TCE, resulted in a concentration dependent decline in the clonogenicity of HeLa cells as evidenced by a continuous decline in the cell survival and a lowest surviving fraction (SF) of 0.25 was obtained for 8 μg/ml (Figure 2). The effect of TCE was greater than that of DMSO treatment at all concentrations. The IC50 was found to be approximately 5.2 μg/ml.
Biological response
To find out the relationship between the cell survival and DNA damage, the cell survival has been plotted on X-whereas the DNA damage on Y-axes, respectively. The increasing DNA damage resulted in a corresponding reduction in the cell survival indicating an inverse relationship between the cell survival and DNA damage. This relationship between cell survival and DNA damage was linear quadratic (Figure 3).
Discussion
The botanicals and natural products have been traditionally accepted as remedies due to the popular belief that they produce fewer adverse side effects [2,38]. Therefore, understanding the potential beneficial or adverse effects of botanicals and natural products extensively used by human population is very important as a public health safety measure. Despite the fact that the drug resistance is a common and major impediment for the successful treatment of cancer by chemotherapy, it is generally not possible to predict the degree or timing of the onset of tumor resistance in most chemotherapy regimens. The comet assay or single cell gel electrophoresis (SCGE) has become one of the standard methods for assessment of DNA damage, with applications in genotoxicity testing, human biomonitoring, molecular epidemiology as well as fundamental research in DNA damage and repair [36]. Recent developments in the assessment of DNA damage by comet assay at the single-cell level suggest that this technique might provide a method for identifying the potential to monitor tumor cell responsiveness to many anticancer agents in situ [43]. In principle, this assay could be applied to any accessible tumor being treated with chemotherapeutic agents that cause overt DNA damage [44]. The comet assay or SCGE has been used for rapid detection and quantitation of DNA damage in individual cells [36,45]. This assay is based on the alkaline lysis of labile DNA at the sites of DNA damage, where the cells are immobilized in a thin agarose gel matrix on slides, gentle lysis and application of electric current to agarose matrix during electrophoresis. The application of electric current causes unwound relaxed DNA to migrate out of the confines of the cell nucleus, which can be visualized after staining with a nucleic acid fluorochrome. The cells that have been inflicted with DNA damage appear as fluorescent comets, with tails of DNA fragmentation or unwinding [36]. In the present study, the potential of dichloromethane extract of giloe (T. cordifolia) in inducing DNA damage was investigated in cultured HeLa cells.
Estimation of DNA damage revealed a concentration dependent rise in the DNA damage and a maximum DNA damage could be discerned in cells treated with TCE for 4 h duration in comparison to those treated only for two h. Therefore, this treatment time was chosen for further studies. The increased DNA migration in HeLa cells observed after 4 h TCE treatment may be likely a sum of genotoxicity or cytotoxicity induced by TCE. Earlier studies have shown that TCE treatment led to an increase in the frequency of micronuclei and chromosome aberrations in HeLa cells in a concentration dependent manner and the present results conforms to our earlier findings that cytotoxic effect of TCE may be due to the damage to cellular genome at molecular level [25,28,38]. The berberine an isoquinoline alkaloid isolated from TCE has been found to increase molecular DNA damage up to 4 h post treatment in HeLa cells earlier [46]. Similarly, Agaricus blazei, a mushroom has been reported to increase DNA damage up to 4 h post-treatment earlier in rats [47]. TCE treatment resulted in a concentration dependent elevation in DNA damage in HeLa cells and the lowest concentration of 1 μg/ ml TCE increased the baseline DNA damage approximately by 10 folds. This rise in the DNA damage is directly related to the decline in cell survival in the present study. The increasing DNA damage by TCE in HeLa cells caused a corresponding reduction in the cell survival and a maximum reduction in the survival was observed for 8 μg/ml TCE, which induced a highest amount of DNA damage. The concentration dependent induction of DNA damage by TCE subsequently resulted in a corresponding decline in the cell survival of HeLa cells in the present study. An identical observation has been reported earlier where increasing OTM (DNA damage) was accompanied by a corresponding decline in the cell survival [48-51]. Berberine has been also reported to accentuate the DNA damage in a concentration dependent manner in HeLa cells [46]. The assessment of DNA damage in HeLa cells at various post-TCE treatment times revealed a time dependent elevation in the DNA damage up to 10 h post-treatment and a marginal but non-significant decline thereafter up to 24 h posttreatment, the last post-treatment time evaluated. This indicates that HeLa cells treated with various concentrations of TCE made a futile attempt to repair DNA damage at 10 h post-treatment as the differences between 10 and 24 h post-treatment were statistically non-significant. An identical effect has been reported with berberine alkaloid present in the TCE [46]. Bleomycin treatment also continuously increased the OTM without signs of repair of damaged DNA in V79 cells up to 6 h post-treatment [41]. In the present study, cells with damaged DNA displayed increased migration of DNA fragments (comet tail) from the nucleus (comet head), which may also be a feature of DNA fragmentation associated with the necrotic/apoptotic cell death [45,49]. In contrast, earlier studies have reported repair of DNA damage after 1 h post-treatment in rats treated with Agaricus blazei and bladder cancer cell lines after irradiation [44,47-49].
The evaluation of biological response gives a direct indication of association of DNA damage with cytotoxicity and a direct correlation between the DNA damage and cell death has been observed in the present study [28,30,31,40]. TCE treatment has been reported to increase the radiation-induced molecular DNA damage and subsequently reduce the survival of HeLa cells earlier [31]. Berberine has been shown to exhibit identical relationship between the DNA damage and cell survival [46]. Similarly, the cell survival and DNA damage has been reported to be inversely correlated in V79 cells treated with belomycin [40]. A similar effect has been reported for micronuclei induction (a biomarker of DNA damage) and cell death earlier [25,28,52-55].
The exact mechanism of induction of DNA damage by TCE is not known. The increased DNA damage may not be ascribed to a single mechanism however, several putative mechanisms may have been responsible for the induction of DNA damage by TCE. TCE may have induced free radicals and reduced the antioxidant status of cells by generation of reactive oxygen species (ROS) causing damage to cellular DNA. These free radicals might have caused damage to DNA by hydrolysis, oxidation and electrophilic attack. Giloe has been reported to increase superoxide radicals, and hydrogen peroxide and TNFα [15]. The induction of lipid peroxidation in HeLa cells exposed to TCE may be another mechanism of induction of DNA damage, where the lipid peroxides thus produced would have attacked the molecular DNA leading to greater damage to the cellular DNA [56,57]. TCE has been found to increase lipid peroxidation in cultured HeLa cells earlier [29]. Berberine, an alkaloid is a major constituent of TCE and it has been reported to cause internucleosomal DNA fragmentation, which may have led to the formation of a complex with DNA and inhibit the activity of topoisomerase II enzyme in vitro [58,59]. The importance of topoisomerase II lies in that it acts by passing an intact segment of duplex DNA through a transient double-stranded break, which it generates in a separate double helix and inhibition of topoisomerase II leads to the stabilization of DNA double strand breaks and may subsequently induce cytotoxic effect [60-62]. TCE may have suppressed the topoisomerase II activity causing stabilization of the transient DNA strand breaks, which might have increased the DNA damage and alleviated the survival of HeLa cells in the present study. Teniposide, a potent DNA topoisomerase II inhibitor has been reported to stabilize DNA double strand breaks without allowing them to rejoin leading to cell death and also induce micronuclei in a concentration dependent manner [52,63]. Transcriptional activation of NF-κB and COX-II has been indicated in reduced apoptosis and increased cell survival [64,65]. TCE may have suppressed the expression of NF-κB and COX-II in HeLa, resulting in the reduced cell survival. Recently, berberine, an isoquinoline alkaloid and other phytochemicals present in the extract of giloe have been reported to suppress the transactivation of NF-κB and STAT3 [66,67]. The inhibition of COX-II expression by giloe [68] supports this contention.
It is clear from our study that cytotoxic effects of giloe is due to its ability to induce DNA damage as evidenced by increased OTM. This may be due to the presence of isoquinoline alkaloid berberine, which has been isolated from the methylene chloride extract of giloe (Figure 4). Berberine has been also isolated from giloe earlier [69]. The berberine has been reported to cause DNA damage in HeLa cells [46].
Conclusions
The treatement of HeLa cells with different concentrations of giloe resulted in a concentration dependent increase in the molecular DNA damage and death of HeLa cells. The effective cell killing by TCE may be due to the induction of DNA damage and loss of DNA repair capacity of HeLa cells. The giloe may have produced oxidative stress by augmented formation of free radicals, generation of ROS, induction of lipid peroxides, and its intercalation with nucleosomal DNA. Giloe may have inhibited the action of topoisomerase II resulting in the escalation of DNA damage. Inhibition of STAT3, NF-κB and COX-II may have also contributed to increased DNA damage. This increased DNA damage might have been the leading cause of cytotoxic effect of giloe. The presence of isoquinoline alkaloids berberine as an active component may have contributed to the increased DNA damage and subsequent cytotoxic effects of giole in the present study.