Petrescu Bianca Nausica1,#, B?b?an Anida-Maria1,#, Buh??el Dan1,#, Ionel Anca1,#, Feurdean Nicoleta Claudia1,#, Ilea Aranka1,#, Câmpian Radu Septimiu1,#, Olga Sori??u2,# and Bo?ca Adina Bianca3,#*
1Department of Oral Rehabilitation, Oral Health and Dental Office Management, Iuliu Ha?ieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
2Laboratory of Radiotherapy, Tumor and Radiobiology, Oncological Institute, Cluj-Napoca, Romania
3Faculty of Medicine, Department of Histology, Iuliu Ha?ieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
#All the authors had an equal contribution in this article.
Corresponding Author:
Dr. Bianca Adina Bo?ca
Faculty of Medicine, Department of Histology, Iuliu Ha?ieganu University of Medicine and Pharmacy
Cluj-Napoca, Romania, Str. L. Pasteur, No. 4, Cluj-Napoca, Romania
Tel: +40740248923
E-mail: biancabosca@yahoo.com
Received date: May 29, 2018; Accepted date: June 15, 2018; Published date: June 18, 2018
Citation: Nausica PB, Anida-Maria B, Dan B, Anca I, Claudia FN, et al. (2018) Stem Cells in Head and Neck Cancers Pathogenesis: Are Advanced Glycation End Products (AGEs) Involved? Biochem Mol Biol J 4:12. doi: 10.21767/2471-8084.100061
Keywords
Cancer stem cells; Advanced glycation end products; Dietary advanced glycation end products; Cancer treatment; Carcinogenesis; Targeted therapies
Introduction
Cancer is one of the most difficult to cure diseases and also one of the most frequent causes of death word wide (considered the dominant cause of death in the United States citizens in 2016).
Despite the progress in cancer death prevention over the last 20 years, the statistics still show extremely increased mortality rates due to cancer, which demand continuous efforts for researchers and medical professionals against oncogenesis and its consequences [1]. Although there are numerous methods for treating cancer including surgery, chemotherapy, and radiotherapy [2], which are sometimes combined, cancer prevention and treatment requires more specific methods, based on advanced investigations [3-6].
Literature Review
What are cancer stem cells?
Cancer Stem Cells (CSCs) can be defined as the stem cells of a tumor. Some authors don’t consider CSCs as a separate cell lineage; others describe CSCs as a different type of cells. There is evidence demonstrating the differences between CSCs and common stem cells (SCs). Some authors even state that stem cells are CSCs precursors.
There are some characteristics that distinguish CSCs from other types of cells. One significant property of CSCs is generation of cancer cells, with inexhaustible replication rate. Multiple literature sources agree that CSCs could play an important role in the development of metastases distant from the initial tumor and could also contribute to the recurrence of a tumor after treatment [4,7,8].
Identification and characterization of CSCs vs SCs
The methods for CSCs isolation use the specific surface markers: magnetic cell sorting and flow cytometry [9]. The specific markers are different depending on the type of tumor e.g. CD105 and CD133 for renal cell carcinoma [9].
CD44 positivity and CD24 negativity cells pattern were used for identifying the breast CSCs, but there were some CSCs that could not be identified with this set of markers [10]. CD44 and aldehyde dehydrogenase (ALDH) were found to be positive for head and neck CSCs [11]. ALDH1 is a member of aldehyde dehydrogenase enzymes family useful for differentiating a normal SC from CSCs. Stem cells with high levels of CD44 expression and ALDH1 activity have greater tumorigenic capacity compared with stem cells with low levels [12].
The presence of CD44 marker in head and neck squamous cell carcinoma is related with high tumorigenicity, antiapoptotic function and clonogenic ability, aspects that meet the CSCs criteria. Moreover, CSCs can exhibit features of mesenchymal or epithelial type, according to the expression of CD44 and epithelial specific antigen (ESA). The mesenchymal phenotype CD44high/ESAlow of CSCs is associated with migratory function whereas the epithelial phenotype CD44high/ESAhigh have proliferative activities. Glycogen synthase kinase 3b (GSK3b) is responsible for regulating the self-renewal capacity [12].
Expression of MACC -1 gene is twofold higher in CSCs than in head and neck cancer cells and could be a biomarker for head and neck cancer [13].
Another technique for separating the cell types is MACS (Magnetic-activated Cell Sorting) [9], but the most widely used and the most adjustable method for stem cell identification is flow cytometry [14].
Signaling mechanisms in carcinogenesis involving CSCs
The origin of CSCs has not been yet established [15], even though numerous theories have been described and proposed in the literature. One of the theories sustains that CSCs originate from stem cells [16,17]. This hypothesis is considered accurate due to the common proprieties that the two cell lineages share, such as the ability of self-renewal and differentiation capacity. The difference between CSCs and stem cells is that CSCs have the capacity of developing secondary malignant tumors [16]. Another theory sustains the origin of CSCs from previously differentiated cells which regain their multipotent or pluripotent capacities [16,17]. A third theory suggests that CSCs could originate from the combination of stem cells with cells belonging to other cell lineages [16] (Figure 1).
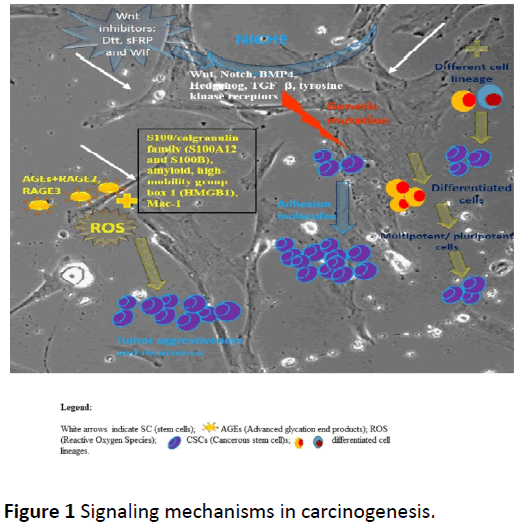
Figure 1: Signaling mechanisms in carcinogenesis.
Some theories imply that the stem cells microenvironment (niche) has a tremendous role in the cells behavior and could maintain a balance regarding the proliferative rate of the cells. The niche that hosts the stem cells produces molecules, such as hh, Wnts, bone morphogenetic proteins (BMP), fibroblast growth factors and Noch, that can influence the cells’ predetermined course [18]. Stem cells may undergo genetic mutations that could block the response to the signals from the microenvironment and, in consequence, the cells separate from the niche’s influence. When the stem cell becomes immune to these control mechanisms, it could undergo unlimited division and even carcinogenesis [18].
There are multiple signaling pathways described in the literature: Wnt, Notch, Hedgehog, TGF-β and tyrosine kinase receptors, all correlated with the guidance of CSCs behavior [16]. For the skin stem cells Wnt signaling has a restraining expansion effect, due to the Wnt inhibitors: Dtt, sFRP and Wif. Wnt can also have a proliferative effect on hematopoietic stem cells, but the mechanism is still unidentified [18]. TGF-β and BMP signaling can also have both proliferating and inhibiting effects on stem cells, but their presence in the microenvironment of the CSCs is uncertain. BMP4 is an exception, since it is found in the niche of the intestinal stem cells adhering to the general rule of the TGF-β signaling, which inhibits cell growth [18]. The effect of the BMP signaling deficit is incontrollable cell proliferation and, consequently, carcinogenesis [18]. The niche also has the capacity to maintain the cells attached due to adhesion molecules: β- catenin and cadherin [18].
Expression of transcription factors in head and neck cancer
The transcription factors which define the CSCs include: Nanog, Oct 3/4, Sox2, Nestin, and CD34 [19]. Aberrant expression of Nanog, Oct 3/4 and Sox2 isoforms and pseudogenes play important roles in tumorigenesis and tumor metastasis, but the mechanisms involved are not completely understood [20].
Etopic expression of Oct 3/4 increases chemoresistance through ABCC6 expression and tumor invasion through Slug expression. According to the higher or lower expression of Oct 3/4 in head and neck squamous carcinoma, the cells express more or less the stemness characteristics. Oct 3/4 enables the distinction between differentiated head and neck squamous carcinoma cells and CSC-like cells [21].
The Nanog transcription factor from CSCs is involved in promoting tumorigenesis, and is also associated with poorer outcome, chemoresistance and epithelial-mesenchymal transition (EMT) which is a crucial event in the metastatic process [22,23]. Other genes expressed in relation with the EMT are: Snail, Slug, Twist, vimentin and fibronectin [23]. ZEB1 and ZEB2 are members of the zinc-finger E-box-binding homeobox factor (ZEB) family. These are transcriptional repressors which induce EMT by suppressing the expression of E-cadherin and thus contribute to the cancer progression.
However, the role of ZEB1 and ZEB2 in mediating CSCs properties in head and neck cancers is still unclear [24].
RhoC is a member of Rho family of GTPases involved in: intracellular signaling, cytoskeletal organization, cell proliferation and regulation of gene expression. RhoC belongs to the Ras superfamily in which some members have been identified with oncogenic activities. RhoC expression was associated with metastasis and genetic mutations in RhoC genes were over-express in head and neck squamous cell carcinoma [25].
Although interleukin 6 (IL-6) contributes, in normal conditions, to host defense in the acute phase responses to injury and infections, several studies demonstrated that IL-6 contributed to the tumorigenic behavior of CSCs [26,27]. Cross talk between endothelial cells and CSC which reside in perivascular niches is crucial for CSCs survival and self-renewal. IL-6 interacts with the IL-6 receptors (IL-6R) and activates Janus kinase (JAK) and STAT3 transcription factor. In head and neck squamous cell carcinoma, IL-6R expression is higher in CSCs than in SCs and the serum levels of IL-6 are correlated with poor patients’ survival [28].
Keratins in head and neck cancer
Keratins are a type of intermediate filament proteins in the cytoskeleton of epithelial cells, which are encoded by 54 functional genes. They contribute to several internal cell processes, such as signaling [29], cell proliferation [30] and stress [31-33]. Keratins’ mutations can lead to keratinopathies; additionally, keratins can be diagnostic markers for proliferative or non-proliferative diseases [34]. Several studies showed that keratins can also be found in the head and neck area (HNA). Their presence has been validated in healthy tissues and also in the soft tissue tumors of the oral cavity. In the HNA, 19 types of keratin (K1 – K19) have been reported (bibliografie - proteomic profile of keratins) [35-39]. Regarding the diagnosis value of keratins, Fulzele et al. showed that K4, K13, K14, K16 and K18 can be used to identify whether a tumor is present or not [33]. Their study was able to establish that K4 and K13 were mainly present in the normal tissue whereas K14, K16 and K17 were detected in the tumor tissue. Futures studies are needed to clarify if CSCs keratins are involved in carcinogenesis.
Integrins in head and neck cancer
Integrins also play an important role in the innovative procedures of fighting against cancer. They are defined as cell surface receptors, involved in the extracellular matrix (ECM) adherence, both in other normal and pathological processes, and this characteristic makes them suitable to be taken into consideration as a potential therapeutic approach in cancer [40,41]. Integrins are involved in cells’ adherence to the ECM, fact that could be incriminated for the resistance to cancer therapy. Through their signals, integrins stimulate the cancer evolution [41]. It was established that through a particular signaling sequence, which includes FAK (Focal Adhesion Kinase) and JNK (c-Jun N-terminal Kinase), beta1-integrins have repercussions on the radioresistence and DNA-proteinkinase dependent non-homologous end joining [41-43]. Koppenhagen et al., in a study on head and neck cancer cells, demonstrated that c-Abl was a significant factor involved in radioresistence of cells derived from solid tumors [41]. Further studies on the communication between CSCs in tumors and with the surrounding tissues through cell surface receptors like integrins (CSCs – CSCs, CSCs – tumoral cells, CSCs - normal surrounding cells and CSCs - tumoral cells - normal surrounding cells), could lead to a better understanding of the cancer spreading mechanisms and local invasion.
Are advanced glycation end products involved in cancer development?
In tumor pathophysiology, numerous determinants are involved. Exogenous and endogenous factors contribute to DNA damage, DNA repair deficiency, somatic mutations and the subsequent progression towards tumor development. Besides the known causes, there is a new approach regarding AGEs (Advanced glycation end products) implication in the evolution and progression of tumors. AGEs are a heterogeneous group of compounds formed by the nonenzymatic reaction between reducing sugars and a free amino or ketone group, found in proteins or lipids. AGEs may have exogenous or endogenous sources and accumulate in timeand dose-dependent manners, leading to a chronical subclinical inflammatory response. Because of the variety in what concerns the receptors, mediators and ligands, AGEs modulate multiple pathogenic mechanisms, including carcinogenesis (Figure 1). Of the AGEs family, Van Heijst found CML and argpyrimidine in human cancer tissues [44]. AGEs accumulation in collagen fibers leads to a deleterious effect, depending on the type of soft or hard structure. Studies have shown a strong correlation between salivary AGEs and bone metastasis in patients with multiple myeloma [45,46]. Vlkova´ and co. evaluated in 16 premalignant lesions (leukoplakia, erythroplakia and lichen planus) ROS salivary markers and found 80% higher AGEs levels compared with healthy patients. The authors supposed the results might be due to the interaction between the carbonyl and oxidative stress [47]. AGEs receptor family includes surface receptors- RAGE, proinflammatory mediators, such as S100/calgranulin family (S100A12 and S100B), amyloid, high-mobility group box 1 (HMGB1), Mac-1. Sanders et al. evaluated the influence of RAGE mediated inflammation of gingival carcinoma cells caused by tobacco exposure and found significant increase of RAGE, IL-6 and IL-8 after 6 six hours exposure to tobacco [48]. Moreover, they tested the reverse effect of semi-synthetic glycosaminoglycans ethers – SAGEs, which reduced glycation compounds in tested samples. Chapman et al. used oral squamous carcinoma cells (OSCC) to evaluate the cellular response induced by RAGE, during cigar smoke. Their findings showed that smoke exposure increased RAGE expression and tumor invasion; removal of exposure factor with the inhibition of RAGE, increased SAGEs in OSCC cells, and reduced smoke- RAGE related invasion [49].
AGE-RAGE interaction leads to mutual molecular increase, reactive oxygen species (ROS) and a continuous inflammatory response [50]. The authors found that AGE-RAGE connection increased vascular inflammation, angiogenesis and thrombogenesis. They also showed that AGE2, AGE3 (glyceraldehyde- and glycolaldehyde derived AGE) were found in human G361 and A375 melanoma cells, and they promoted growth and migration of tumor cells. Bhawal and co. evaluated the role of RAGE in OSCC progression [51] by using primary and metastatic site cells cultures. Their results showed that RAGE mRNA was detected in all primary and metastatic tumors, while RAGE was overall expressed in metastatic tumors. The authors also used invasion assays, to find that after 12 hours, RAGE was present in a higher amount in type IV collagen cell invasion. Tateno et al. analyzed the expression of RAGE in 216 esophageal carcinoma specimens [52].
They found 50% RAGE-positive tumor cells, in cell membrane and cytoplasm. The RAGE levels were negatively correlated with the depth and venous invasion. Landesberg and coworkers evaluated RAGE expression in 38 oral squamous cell carcinoma samples. They found positive correlation regarding the histological differentiation, the RAGE expression being more reduced with the degree of differentiation [53]. Sasahira’s research investigated RAGE expression in 74 OSCC specimens, and their correlation with clinical and pathological parameters. They found a highly positive connection between RAGE and the depth of tumor invasion, but inversely with the degree of histological differentiation [54]. The same authors analyzed RAGE implication in tumor OSCC angiogenesis [55]. They correlated RAGE with microvessel density (MVD) and vascular endothelial growth factor (VEGF) with the tumor progression. When HMGB1 was added to RAGE, VEGF secretion was elevated as well, the results suggesting RAGE role in tumoral angiogenesis.
Antioxidant effect is modulated by nuclear factor-erythroid 2-related factor 2 (Nrf-2), also involved in p53 apoptotic mediated response. An in vitro study [56] showed that cell exposed to AGEs increased ERK (extracellular-signal-regulated kinase) phosphorylation, which lead to Nrf-2 downregulation, suppression of p53 protection gene, which could explain the degree of invasiveness of tumor cells. Another in vitro study [57] analyzed the effect of HMGB1 in progression and invasion of human nasopharyngeal carcinoma cell line (HONE-1). They also used assays to evaluate the cells metastatic capacity. The research team demonstrated that suppression of HMGB1 downregulated RAGE expression and ERK dependent process, which inhibited all malignant HONE-1 cells properties of proliferation, migration and invasion.
A systematic review investigated the HMGB1-RAGE inflammatory interaction in what concerns head and neck melanoma pathology [58]. Intratumoral RAGE and HMGB1 were found to be overexpressed compared to control ones, whereas HMGB1 levels were positively correlated with the aggressiveness of melanoma.
Because of the present methods used in cooking food, the Western diet which has been spread worldwide, habits in bringing microwaves rather than classic heating, dietary AGEs (DAGEs) could be as well, as an exogenous source, an etiological factor in carcinogenesis. Depending on the intake, DAGEs exert their continuous negative effect. Studies have been made on oral cavity biofluids and biological ones, to determine alterations associated with different pathologies. Saliva, as an easy to harvest fluid, could be used to provide information regarding AGEs, DAGEs and their correlation with the state of tumor progression. A salivary biosensor could be used for screening and as diagnosis device or for real-time AGEs monitoring [59]. The tool might be useful to evaluate salivary AGEs in correlation with the cancer relapse after surgical excision, chemotherapy or both, as a non-invasive and easy to use device.
The above studies contribute with new evidence which suggest an AGEs contribution in etiology and progression of malignant lesions which should be taken into account. Future treatment strategies could target AGE-RAGE axis for a superior efficiency and disease prognosis. Moreover, studies on RAGE expression in CSCs subpopulation of head and neck cancer tissues could represent a future approach in targeting CSCs for cancer treatment.
New Challenges in Targeting Cscs and Ages in Cancer Treatment
While in 2013, there was only an aspiration for identifying stem cells by using their specific markers and there was not enough data proving that CSCs existed in some forms of cancer, the researchers had a hope that in perspective, the metastasis and recurrence of cancer could be prevented only by targeting the CSC populations [18]. Researches acknowledged the importance using CSCs as an objective for trying to treat many types of cancer, among them being the head and neck researchers, who suggested not only targeting CSCs, but also their microenvironment, stimulating apoptosis by sending extrinsic signals to the cells [16].
Discussion
Futures research is needed in order to assess the involvement of CSCs keratins in carcinogenesis and to clarify if cellular stress induced by keratins is added (even have potentiation or augmentation effect) to AGEs (Advanced glycation end products) cellular damages. Another question would be if by controlling dietary AGEs intake, we could prevent cancer development. Blocking CSCs surface receptors like integrins, probably will enable the prevention of cancer relapses.
Additional questions arise and require further studies: Could AGEs receptor family such as surface receptors – RAGE interact with integrins in cancer cells and/or CSCs? Could RAGE blocking be useful in targeting the treatments toward CSCs? Could blockage of AGEs accumulation in collagen fibers prevent local tumor invasion? Could salivary AGEs assessment be a useful tool in the follow up of cancer patients with relapse and metastasis or in the assessment of the risk in cancer development? Could also be useful the salivary IL-6 and IL-8 evaluation? Could dietary AGEs be involved in carcinogenesis?
These are some of the questions that still need to be addressed in the future research.
Conclusion
In head and neck disorders, various pathogenic mechanisms are involved. Besides stem cells abnormalities, exogenous and endogenous factors are implicated in either the initiation or in the aggravation of tumor process. A therapeutic approach focused on targeting CSCs in head and neck cancers multiple pathogenic mechanisms simultaneously or sequentially, such as receptors (integrins and RAGE) or/and intacellular structures (keratins) and transcriptional factors will probably enable a better management of these diseases.
AGEs, mainly dietary ones, by their time and dosedependent continuous accumulation, could be an important element in the exacerbation and spreading of cancerous lesions. RAGE-AGEs interaction generates chronic inflammation, promotes ROS formation and activates other deleterious cells and pathways leading uncontrolled cell division. Since the AGEs amount is directly related to the aggressiveness and state of tumor lesions, a salivary biosensor could be an innovative method for the prognosis and monitoring of cancerous cells pathology. Besides classical surgical excision, chemotherapy and radiotherapy, it would represent a non-invasive therapy for completing the protocol in head and neck cancer pathology.
While trying to develop new ways of curing cancer, the pathogenic mechanisms need to be considered. Thus, the treatment will be more targeted and, hopefully, the patients’ survival could be improved.
Acknowledgements
This study was supported by the Doctoral Research Projects (PCD 2016) of “Iuliu Ha?ieganu” University of Medicine and Pharmacy Cluj-Napoca, No. 7690/15.04.2016, PhD Grant of “Iuliu Ha?ieganu” University of Medicine and Pharmacy Cluj- Napoca, No 3999/01.10.2016 and partially by the COFUNDERA- HDHL ERANET Project, European and International Cooperation - Subprogram 3.2 - Horizon 2020, PNCDI III Program - Biomarkers for Nutrition and Health – “Innovative technological approaches for validation of salivary AGEs as novel biomarkers in evaluation of risk factors in diet-related diseases”, grant no 25/1.09.2017.
Conflict of Interests
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article. The authors have no any financial interests (direct or indirect) with respect to publication of this article.
References
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics. CA Cancer J Clin 66: 7-30.
- Ramaswami R, Harding V, Newsom-Davis T (2013) Novel cancer therapies: Treatments driven by tumor biology. Postgrad Med J 89: 652-658.
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, et al. (2016) Cancer treatment and survivorship statistics. CA Cancer J Clin 66: 271-289.
- Ajani JA, Song S, Hochster HS, Steinberg IB (2015) Cancer stem cells: The promise and the potential. Semin Oncol 42: 17.
- Touil Y, Igoudjil W, Corvaisier M, Stechly L, Skrypek N, et al. (2015) HHS Public Access 20: 837-846.
- Tsimberidou A (2015) Targeted therapy in cancer. Cancer Chemother Pharmacol 76: 1113-1132.
- Tang DG (2012) Understanding cancer stem cell heterogeneity and plasticity. Cell Res 22: 457-472.
- Chen K, Huang Y, Chen J (2013) Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 34: 732-740.
- Khan MI, Czarnecka AM, Helbrecht I, Bartnik E, Lian F, et al. (2015) Current approaches in identification and isolation of human renal cell carcinoma cancer stem cells. Stem Cell Res Ther 6: 178.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci 100: 3983-3988.
- Kobayashi NC, Noronha SM (2015) Cancer stem cells: A new approach to tumor development. Rev Assoc Med Bras 61: 86-93.
- Seino S, Shigeishi H, Hashikata M, Higashikawa K, Tobiume K, et al. (2016) CD44(high) /ALDH1(high) head and neck squamous cell carcinoma cells exhibit mesenchymal characteristics and GSK3β-dependent cancer stem cell properties. J Oral Pathol Med 45(3): 180-188.
- Evran E, ?ahin H, Akba? K, Çi?dem S, Gündüz E (2016) Investigation of MACC1 gene expression in head and neck cancer and cancer stem cells. Clin Invest Med 39: 27506.
- Wilson GD, Marples B, Galoforo S, Geddes TJ, Thibodeau BJ, et al. (2013) Isolation and genomic characterization of stem cells in head and neck cancer. Head Neck 35: 1573-1582.
- Vincent A, Van Seuningen I (2012) On the epigenetic origin of cancer stem cells. Biochim Biophys Acta - Rev Cancer 1826: 83-88.
- Yu Z, Pestell TG, Lisanti MP, Pestell RG (2013) Cancer stem cells. Biochem Cell Biol 44: 2144-2151.
- Li L, Neaves WB (2006) Normal stem cells and cancer stem cells: The niche matters. Cancer Res 66: 4553-4557.
- Singh R, Chen C, Phelps RG, Elston DM (2014) Stem cells in the skin and their role in oncogenesis. J Eur Acad Dermatology Venereol 28: 542-549.
- Apostolou P, Toloudi M, Chatziioannou M, Ioannou E, Papasotiriou I (2012) Cancer stem cells stemness transcription factors expression correlates with breast cancer disease stage. Curr Stem Cell Res Ther 7: 415-419.
- Liu A, Yu X, Liu S (2013) Pluripotency transcription factors and cancer stem cells: Small genes make a big difference. Chin J Cancer 32: 483-487.
- Koo BS, Lee SH, Kim JM, Huang S, Kim SH, et al. (2015) Oct4 is a critical regulator of stemness in head and neck squamous carcinoma cells. Oncogene 34: 2317-2324.
- Iv Santaliz-Ruiz LE, Xie X, Old M, Teknos TN, Pan Q (2014) Emerging role of nanog in tumorigenesis and cancer stem cells. Int J Cancer 135: 2741-2748.
- Chen YS, Huang WL, Chang SH, Chang KW, Kao SY, et al. (2013) Enhanced filopodium formation and stem-like phenotypes in a novel metastatic head and neck cancer cell model. Oncol Rep 30: 2829-2837.
- Chu PY, Hu FW, Yu CC, Tsai LL, Yu CH, et al. (2013) Epithelial-mesenchymal transition transcription factor ZEB1/ZEB2 co-expression predicts poorprognosis and maintains tumor-initiating properties in head and neck cancer. Oral Oncol 49: 34-41.
- Islam M, Sharma S, Teknos TN (2014) RhoC regulates cancer stem cells in head and neck squamous cell carcinoma by overexpressing IL-6 and phosphorylation of STAT3. PLoS One 9: e88527.
- Sansone P, Storci G,Tavolari S, Guarnieri T, Giovannini C, et al. (2007) IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest 117: 3988-4002.
- Korkaya H, Kim G, Davis A, Malik F, Henry NL, et al. (2012) Activation of an IL-6 inflammatory loop mediates trastuzumab resistance in her 2+ breast cancer by expanding the cancer stem cell population. Mol Cell 47: 570-584.
- Nör C, Zhang Z, Warner KA, Bernardi L, Visioli F, et al. (2014) Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 16: 137-146.
- Alam H, Kundu ST, Dalal SN, Vaidya MM (2011) Loss of keratins 8 and 18 leads to alterations in alpha-6-beta4-integrin-mediated signaling and decreased neoplastic progression in an oral-tumor-derived cell line. J Cell Sci 124: 2096-2106.
- Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM (2011) Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell 22: 4068-4078.
- Denk H, Stumptner C, Zatloukal K (2000) Mallory bodies revisited. J Hepatol 32: 689-702.
- Cadrin M, Hovington H, Marceau N, Mc Farlane-Anderson N (2000) Early perturbations in keratin and actin gene expression and fibrillar organisation in griseofulvin-fed mouse liver. J Hepatol 33: 199-207.
- Fulzele A, Malgundkar SA, Govekar RB, Patil A, Kane SV, et al. (2013) Proteomic profile of keratins in cancer of the gingivo buccal complex: Consolidating insights for clinical applications. J Proteomics 91: 242-258.
- Toivola DM, Boor P, Alam C, Strnad P (2015) Keratins in health and disease. Curr Opin Cell Biol 32: 73-81.
- Chen J, He QY, Yuen AP, Chiu JF (2004) Proteomics of buccal squamous cell carcinoma: The involvement of multiple pathways in tumorigenesis. Proteomics 4: 2465-2475.
- Baker H, Patel V, Molinolo AA, Shillitoe EJ, Ensley JF, et al. (2005) Proteome-wide analysis of head and neck squamous cell carcinomas using laser-capture microdissection and tandem mass spectrometry. Oral Oncol 41: 183-199.
- Patel V, Hood B, Molinolo A, Lee N, Conrads T, et al. (2008) Proteomic analysis of laser-captured paraffin-embedded tissues: A molecular portrait of head and neck cancer progression. Clin Cancer Res 14: 1002-1014.
- Schaaij-Visser TBM, Graveland AP, Gauci S, Braakhuis BJM, Buijze M, et al. (2009) Differential proteomics identifies protein biomarkers that predict local relapse of head and neck squamous cell carcinomas. Clin Cancer Res 15: 7666-7675.
- Thiel UJ, Feltens R, Adryan B, Gieringer R, Brochhausen C, et al. (2011) Analysis of differentially expressed proteins in oral squamous cell carcinoma by MALDI–TOF MS. J Oral Pathol Med 40: 369-379.
- Kapp TG, Rechenmacher F, Sobahi TR, Kessler H (2013) Integrin modulators: A patent review. Expert Opin Ther Pat 23: 1273-1295.
- Koppenhagen P, Dickreuter E, Cordes N (2017) Head and neck cancer cell radio-sensitization upon dual targeting of c-Abl and beta1-integrin. Radiother Oncol 124: 370-378.
- Dickreuter E, Eke I, Krause M, Borgmann K, Van Vugt MA, et al. (2016) Targeting of b1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene 35: 1353-1362.
- Storch K, Eke I, Borgmann K, Krause M, Richter C, et al. (2010) Three dimensional cell growth confers radio-resistance by chromatin density modification. Cancer Res 70: 3925-3934.
- Van Heijst JWJ, Niessen HWM, Hoekman K, Schalkwijk CG (2005) Advanced glycation end products in human cancer tissues: Detection of N{epsilon}-[carboxymethyl]lysine and argpyrimidine. Ann NY Acad Sci 1043: 725-733.
- Gangemi S, Allegra A, Alonci A, Cristani M, Russo S, et al (2012) Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm Res 61: 1063-1067.
- Katz J, Moreb J, Baitinger C, Singer C, Caudle RM (2017) Advanced glycation end products (AGEs) in saliva of patients with multiple myeloma - a pilot study. Leukemia & Lymphoma. Informa Healthcare USA, Inc, 58: 934-2938.
- Vlková B, Stanko P, Minárik G, Tóthová L, Szemes T, et al. (2012) Salivary markers of oxidative stress in patients with oral premalignant lesions. Arch Oral Biol 57: 1651-1656.
- Sanders NT, Dutson DJ, Durrant JW, Lewis JB, Wilcox SH, et al. (2017) Cigarette Smoke Extract (CSE) induces RAGE-mediated inflammation in the Ca9-22 gingival carcinoma epithelial cell line. Arch Oral Biol 80: 95-100.
- Chapman S, Mick M, Hall P, Mejia C, Sue S, et al. (2018) Cigarette smoke extract induces oral squamous cell carcinoma cell invasion in a receptor for advanced glycation end-products-dependent manner. Eur J Oral Sci 126: 33-40.
- Abe R, Yamagishi S (2008) AGE-RAGE system and carcinogenesis. Curr Pharm Des 14: 940-945.
- Bhawal UK, Ozaki Y, Nishimura M, Sugiyama M, Sasahira T, et al. (2005) Association of expression of receptor for advanced glycation end products and invasive activity of oral squamous cell carcinoma. Oncol 69: 246-255.
- Tateno T, Ueno S, Hiwatashi K, Matsumoto M, Okumura H, et al. (2009) Expression of Receptor for Advanced Glycation End products (RAGE) is related to prognosis in patients with esophageal squamous cell Carcinoma. Ann Surg Oncol 16: 440-446.
- Landesberg R, Woo V, Huang L, Cozin M, Lu Y, et al. (2008) The expression of the receptor for glycation end products (RAGE) in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105(5): 617-624.
- Sasahira T, Kirita T, Bhawal UK, Yamamoto K, Ohmori H, et al. (2007) Receptor for advanced glycation end products (RAGE) is important in the prediction of recurrence in human oral squamous cell carcinoma. Histopathology 51(2): 166-172.
- Sasahira T, Kirita T, Bhawal UK, Ikeda M, Nagasawa A, et al. (2007) The expression of receptor for advanced glycation end products is associated with angiogenesis in human oral squamous cell carcinoma. Virchows Archiv 450: 287-295.
- Ko S, Ko HA, Shieh TM, Chi TC, Chen HI, et al. (2017) Advanced glycation end products influence oral cancer cell survival via Bcl-xl and Nrf-2 regulation in vitro. Oncol Lett 13: 3328-3334.
- Peng T, Hu M, Wu T, Chen Z, Zhang C, et al. (2015) Effects of high-mobility group box 1 knockdown on proliferation, migration and invasion of the HONE-1 human nasopharyngeal carcinoma cell line. Mol Med Rep 12: 7531-7537.
- Nguyen AH, Detty SQ, Agrawal D (2017) Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review. Anticancer Res 37: 1-8.
- Ilea A, B?b?an AM, Bo?ca BA, Cri?an M, Petrescu NB, et al. (2018) Advanced glycation end products (AGEs) in oral pathology. Arch Oral Biol 93: 22-30.


