Research Article - (2017) Volume 3, Issue 1
Seghier Abdelkarim1*, Hadjel Mohammed1 and Benderdouche Nouredine2
1 Department of Industrial Organic Chemistry, University of Science and Technology - Mohamed BOUDIAF of Oran, Oran, Algeria
2 Laboratory of Structure, Elaboration and Application of Molecular Materials (SEA2M), University of Mostaganem, Mostaganem, Algeria
*Corresponding Author:
Seghier Abdelkarim
Laboratory of Sciences, Technologies
and Process Engineering, Department
of Industrial Organic Chemistry, Faculty
of Chemistry, University of Science and
Technology - Mohamed BOUDIAF of Oran,
BP 1505 El Mnaouar, Bir El Djir 31000 Oran,
Algeria.
Tel +213 542161512
E-mail: krimouvert@yahoo.fr, abdelkarim.seghier@univ-usto.dz
Received date: April 12, 2017; Accepted date: May 02, 2017; Published date: May 08, 2017
Citation: Abdelkarim S, Mohammed H, Nouredine B. Sorption of Methylene Blue Dye from Aqueous Solution Using an Agricultural Waste. Trends Green Chem. 2017, 3:4. doi: 10.21767/2471-9889.100017
Millions of tons of prickly (peel) bark of cactus fruit (PBCF) are produced per year. This work is to use this agricultural waste as a low cost biomaterial for the treatment of water contaminated with organic pollutant. PBCF has been characterized by several analytical techniques i.e. SEM, EDS and FTIR spectroscopy. In addition, the determination of iodine value which gives information on area surface. PBCF were used to remove Methylene blue (MB) from the aqueous solution. Experiments were carried out as function of contact time, initial dose of PBCF, initial pH aqueous solution, initial concentration of MB (90-2000 mg L-1), and temperature (20-40°C). The pseudo-second-order was the best applicable model to describe the sorption of MB by Prickly bark of cactus fruit. The equilibrium sorption data of Methylene blue by Prickly barks of cactus fruit were analysed by Langmuir and Freundlich models. The preliminary results indicate that the Langmuir model provides the best correlation of the experimental data with an important capacity of sorption of Methylene blue onto Prickly bark of cactus fruit (222.22 mg g-1). Moreover the parameters of the Freundlich model showing that the sorption of MB onto PBCF is favourable. Finally, the sorption capacity of PBCF is compared to those of other sorbents materials reported in the literature.
Keywords
Sorption; Basic dye; Bio-sorbent; Isotherms; Agricultural waste
Introduction
Dyes have been excessively released into the environment due to rapid industrialization and have created a major global concern. They are chemical compounds which can connect themselves to surfaces or fabrics to impart colour. The majority of dyes are complex organic molecules and are required to be resistant to many things such as the action of detergents [1]. They are often found in industrial wastewaters, which originate from textile, leather tanning, paper, cosmetics, plastics, rubber, food processing, manufacturing and printing dye activities [1].
Removal of dyes from industrial wastewater is an important problem due to the characteristics of these dyes, as that they are non-degradable and therefore persistent [2]. Moreover, most of the pollutants are toxic to living organisms and they have a large influence on the photosynthetic activity in aquatic biota [3]. To respect the environmental norms, the toxic materials should be removed from wastewater before its disposal [4].
Methylene blue is one of the most dyes found in the waters discharged by industrials activities and histologic microbiologic [5]. Severe toxicity is imposed by this pollutant: toxicity in premature neonate [5] and toxicity on the central nervous system [6]. To deal with the problems caused by the dyes including Methylene Blue, several studies were devoted to their elimination such as: adsorption on activated carbon [7-9], coagulation/flocculation [10,11], poly-electrolyte promoted [12,13], ozonation [14], nano-filtration [15-17], photo-catalytic degradation in advanced oxidation process [18,19], electrochemical treatment [20,21] and biological treatment in particularly enzyme [22-31].
According to the literature, adsorption on activated carbon remain among the most and easy technical to implement [32]. Several studies have been carried out in this area, namely: cotton [33], corn [34], silk worm cocoon [35].
However, the activation (chemical or physical) of carbon increases the cost of treatment; also their regeneration is a delicate operation [36].
To this end, new biomaterials subject of much research in recent years such as the adsorption of Methylene Blue on biomaterial using cactus tree [2]. As yet this technique was not applied to prickly bark of cactus fruit (peel), hence never reported in the literature according to our knowledge.
Experimental
In this work prickly bark of cactus fruit was used as bio sorbent. The raw material was dried in sunlight for 15 days at an average daylight temperature of 40°C, watched several times with distilled water, dried at 40°C, grounded to powder and sieved conserving only particles having a seize not exceeding 315 nm. The product obtained noted (PBCF). To study the morphology of the PBCF surface its structure was observed using a HIROX SH 400 M SEM-EDS BRUKER apparatus. To detect the functionality present in (PBCF), FTIR spectroscopy studies using the PerkinElmer Spectrum Two FTIR with UATR sampling accessory. The sorption capacity of materials to the dyes can be examined by calculating the iodine value [37]. Generally adsorbents with a high iodine number have a high surface area and are suitable for adsorbing small compounds [37]. For this propose the PBCF surface area was studied by measuring their iodine number (mg g-1) according to the standard procedure [38] by using the 0.1 N standardized iodine solution. Sample volumes of 100 ml of the iodine solution were treated with 1 g of the material. After equilibrium, the remaining iodine in the supernatants was titrated with 0.1 N sodium thiosulfate solution. The Iodine Number was reported as the amount of iodine adsorbed per gram of adsorbent at a residual iodine concentration of 0.02 N.
The 90 g/L concentration Methylene blue solution (initial pH) used to determine the equilibrium contact time. Batch process was carried out at 20 ± 2°C (ambient laboratory temperature). 25 ml of Methylene blue solution was separately mixed with 0.1 g of the Prickly bark of cactus fruit (PBCF). The mixtures were put into the shaking batch (400 rev/min shaking rate) during different time intervals, ranging from 10 to 360 min, then the solids particles were removed by centrifugation then the Methylene blue concentration in aqueous solution was determined with a SPECORD 200 PLUS –ANALYTIK, JENA- UVvisible spectrophotometer at 664 nm using a cuvette of 10 mm wide. The sorption capacities of MB onto PBCF were calculated by [39]:
qt = (C0-Ce) V/W (1)
Where qt is the sorption capacity (mg g-1) at any time; C0 and Ce (mg L-1) are respectively the initial and final adsorbate concentration; V is the volume of the aqueous solution (L); W is the mass of the bio sorbent used (g).
Under the same operating conditions (initial pH, ambient laboratory temperature and 400 rpm shaking rate) , effect of PBCF mass on MB percentage removal at 100 mg L-1 and 0.05-0.3 g of adsorbent were studied.
Under the same operating conditions (initial pH, ambient laboratory temperature and 400 rpm shaking rate) , effect of PBCF mass on MB percentage removal at 100 mg L-1 and 0.05-0.3 g of adsorbent were studied.
The isotherm of bio sorption experiments were performed in a rotary shaker at 400 rpm using 250 ml Erlenmeyer flasks containing 25 ml of different MB concentrations at initial pH. After 60 min of contact (according to the kinetic study), with 0.1 g PBCF biomass, the concentrations of MB equilibriums and sorption capacities were determined by the same methods and devices already reported beforehand (kinetics study).
These isotherms were studied for temperatures of 20, 30 and 40°C.
Results and Discussion
Characterization of the bio-sorbent
Following SEM image represented by Figure 1, the surface of Prickly bark of cactus fruit is full of cavities distributed on a heterogeneous surface. Similar morphology was on the external surface for cactus tree [2]. Moreover and according the literature [3,37,40], this sorbent has a high surface area view its great iodine index value reaches 436 mg g-1. The elemental composition of the material in particular oxygen and carbon is illustrated by the spectrum EDS of Figure 2. The analysis reveals the presence of oxygenated functional groups.
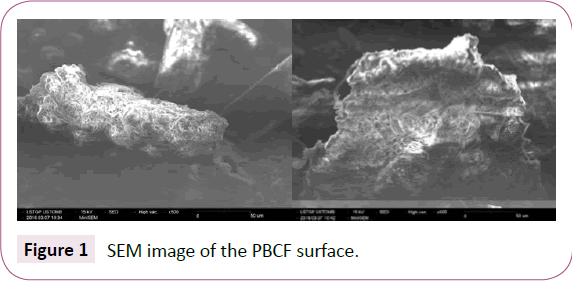
Figure 1: SEM image of the PBCF surface.
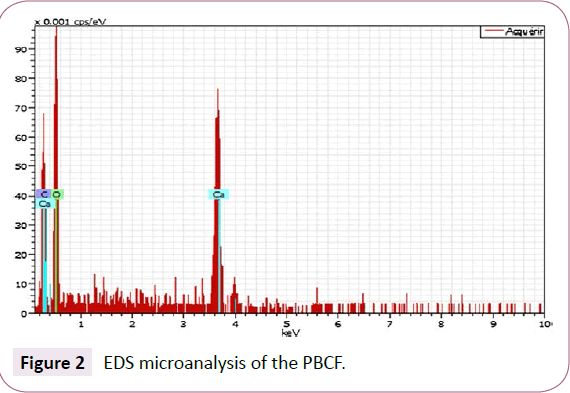
Figure 2: EDS microanalysis of the PBCF.
The ATR-FTIR spectra (Figure 3) indicates an intensive OH peak at 3337.26 cm-1 [3,2], the peaks alkyl at 2850.87 and 2918.03 cm-1 are assigned to aliphatic C-H stretching bonds [3, 41], The bands, at 1616.28 and 1729.61 cm-1 due to the presence of C=O bonds in carboxylate functional groups and carboxylic acid respectively [3,41,42], the peak at 1424.49 , which is identical to the presence of –C-H stretching in saturated aliphatic hydrocarbons (alkanes) with sp3-hybridizations [41], the band to 1374.23 cm-1, can be assigned to the vibration of elongation of the phenolic OH [2], the peak appeared to 1315.72 cm-1 due to C-O stretching in esters, ether, phenol or carboxylic groups [42]. Finally the peaks at 1027.74-1157.27 cm-1 can be ascribed to C-N stretching in aliphatic amines [41].
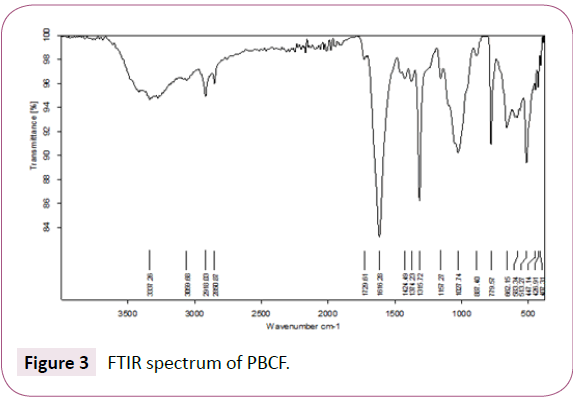
Figure 3: FTIR spectrum of PBCF.
Contact time
The equilibrium time for the sorption of MB onto PBCF (Figure 4) was found to be 60 min. Any intra particular diffusion was noted during the sorption experiments that should lead to a decrease in the slope between the fast initial and the equilibrium phase [3]. While a contact time of 60 min is quite sufficient for the sorption experiments following.
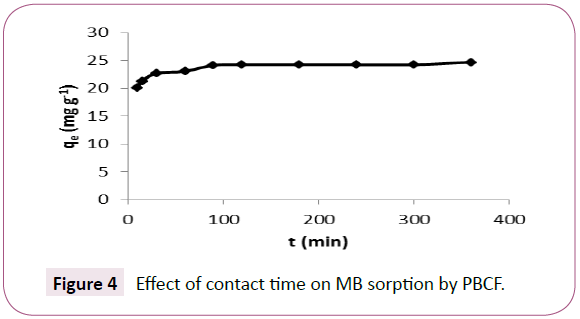
Figure 4: Effect of contact time on MB sorption by PBCF. Figure 5 Pseudo-second-order model for MB removal by PBCF. q
Sorbent rate constant
According to the literature, the first-order kinetic model given by Lagergren and the Ho’s pseudo-second-order model are the most commonly used to provide the mechanism involved in the sorption process [43].
The Lagergren’s first order model [43] is expressed by the following linear equation:
log (qe-qt)=log qe-(k1/2.303) t (2)
Were qe and qt are the amounts (mg g-1) of sorbed dye at instant t and at equilibrium respectively and k1 (l min-1) is the rate constant of sorption (min-1).
The Ho’s pseudo-second-order model [43] is expressed by the linear formula (3):
t/qt=1/K2qe 2+(1/qe) t (3)
K2 (g mg−1 min−1) is the pseudo-second-order rate constant.
The results of this work did not fit the Largergern’s first order model, however the good correlation of the plot (Figure 5) with the experimental results indicate that the sorption of and MB Follow the pseudo second order kinetics. This model is frequently used in several works in the same field [3,43].
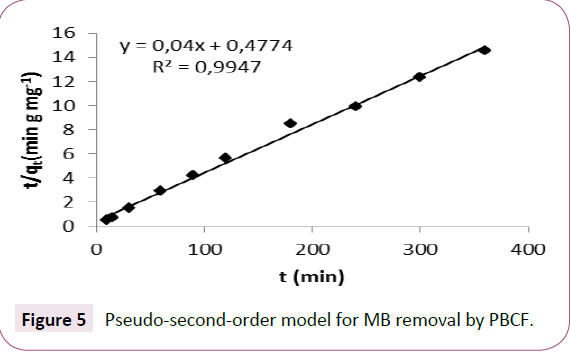
Figure 5: Pseudo-second-order model for MB removal by PBCF.
The parameters of the Ho’s pseudo-second-order model grouped in Table 1 shows a good determination coefficient value (0.99). Moreover the experimental and calculated values of the equilibrium removal capacity of MB onto PBCF were almost equivalent (qe ≈ qe calculated). Same phenomena for MB removal in batch system have been described in the literature [44].
| qe(mg g-1) | K2(gm g-1 min-1) | qe calculated (mg g-1) | R2 |
| 24,6975 | 0,0034 | 25 | 0,9947 |
Table 1: Experimental and calculated rate constants for the second-order sorption.
Effect of sorbent mass
It was noted that the percentage of MB removal increases according the amount of PBCF mass (Figure 6). This finding is justified by the high number of reactive vacant sites the mass transfer and concentration gradient is high. These favour the transfer of MB solute to the external surface of sorbent and increase on its removal percentage [41]. This in term of economic and view the important MB removal capacity, 0.1 g of sorbent was selected for the experiences those suites.
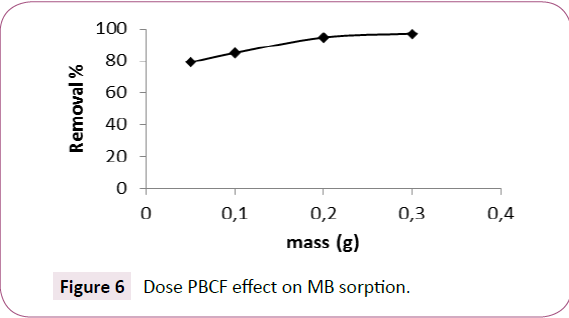
Figure 6: Dose PBCF effect on MB sorption.
Effect of pH
Figure 7 shows that the highest sorption capacities were recorded at pH greater than pHZPC which gives a negative charge to the bio sorbent surface which leads to the increase of the fixation of the dye positively charged. However, the low values of this sorption capacity at pH less than 8 can be explained by the competition of protons with the solute at the available sites on the surface bio sorbent. The same interpretations have been reported by Bestani and Benderdouche et al onto an activated desert plant [37].
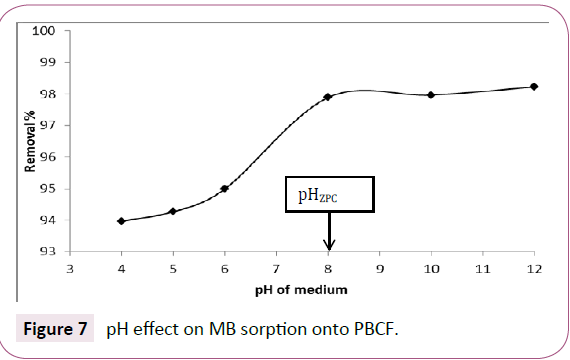
Figure 7: pH effect on MB sorption onto PBCF.
Sorption isotherms
Sorption isotherms data are an important study in the design of the sorption in batch systems.
The Freundlich and Langmuir mathematical models are the most frequently used to predict the equilibrium between the liquid phase (free solution) and the solid phase (adsorbent-attached solute) concentrations [45].
Freundlich isotherm model is represented by the following linear equation [46]:
Log qe=1/n log Ce+log KF (4)
Were qe (mg g-1) is the equilibrium sorption concentration of MB per gram of PBCF; Ce (mg L-1) is the concentration of the solute in solution at equilibrium; KF and n are Freundlich constants which are related to the sorption capacity and the intensity of sorption. In addition the values of n have a relation with favourability of the sorption process [47,48]. The value 1/n ranging between 0 and 1 is a measure of sorption intensity or surface heterogeneity; over this value approach 0 the sorption is more heterogeneous [49].
Langmuir isotherm model is described by the following linear equation [50]:
Ce/qe=Ce/Qmax+1/Qmax KL (5)
qe (mg g-1) is the amount of solute adsorbed per unit dose of sorbent, Ce (mg L-1) is the dye equilibrium concentration, Qmax (mg g-1) is the sorption capacity, KL (L/mg) is the Langmuir constant related to the adsorption energy. Moreover the equilibrium parameter (separation factor) RL is among the most important factors discussed in this paper, is calculated by the fallowing relation [51]:
RL=1/(1+bC0) (6)
Where b is the Langmuir constant and C0 (mg L-1) is the highest solute concentration.
The isotherm to be either unfavorable (RL>1), linear (RL=1), favorable (0L<1) or irreversible (RL=0).
According to the plots (Figures 8 and 9) and the parameters of Freundlich and Langmuir grouped in Table 2, the correlation coefficients indicate a correct fit for both mathematical models (0.9958 and 0.9983 for Freundlich and Langmuir model respectively), however the Langmuir isotherm (Figure 9) fits better the experimental results. The value of n (1.230) indicates that the sorption is favorable. Moreover the RL value between 0 and 1 (0.0142) indicates that the sorption isotherm is favorable. The sorption capacity (222.22 mg g-1) of BM onto PBCF is better than those of many sorbent cited in the literature (Table 3).
| Langmuir constants | Freundlich constants | ||||||
| Qmax(mg g-1) | KL (L mg-1) | R2 | RL | 1/n | n | KF | R2 |
| 222,2222 | 0,0345 | 0,9983 | 0,0142 | 0,813 | 1,230 | 2,212 | 0,9958 |
Table 2: Langmuir and Freundlich constants for Methylene Blue sorption on PBCF.
| Sorbent | Sorption capacity (mg g-1) | Reference |
| inactivated desert plant | 23 | [37] |
| Pyrolized desert plant | 53 | [37] |
| Chemically activated desert plant | 130 | [37] |
| Commercial activated carbon | 200 | [37] |
| Black stone cherries | 321,75 | [52] |
| Walnut shell | 315 | [53] |
| Oil palm shell | 243,90 | [54] |
| Hazelnut husks | 204 | [55] |
| Plant leaf powder | 61,22 | [56] |
| Wood apple rind | 40 | [57] |
| Caulerpalentillifera | 417 | [58] |
| Sargassummuticum | 279,20 | [59] |
| Dead macro fungi (F) | 232,73 | [60] |
| Dead macro fungi (P) | 201,38 | [60] |
| Algae Gelidium | 171 | [61] |
| Algal waste | 104 | [61] |
| Composite material | 74 | [61] |
| Duckweed | 144,93 | [62] |
| Green alga | 40,20 | [63] |
| The brown alga | 38,61 | [64] |
| PBCF | 222,22 | this study |
Table 3: Comparison of sorption capacity of Methylene Blue onto activated carbons and other low-cost sorbents.
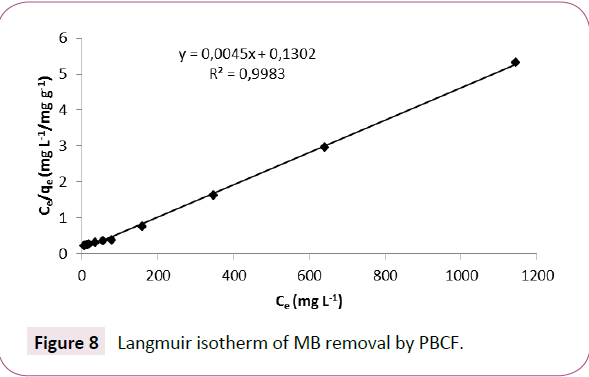
Figure 8: Langmuir isotherm of MB removal by PBCF.
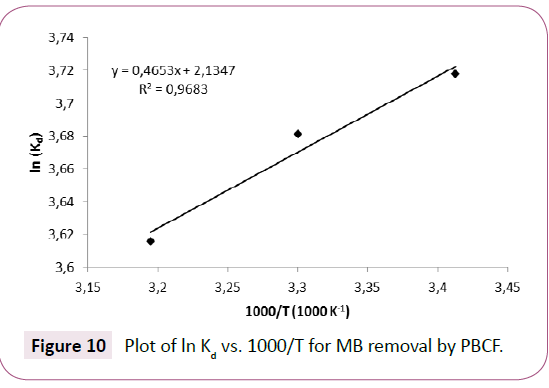
Figure 9: Freundlich isotherm of MB removal by PBCF.
Thermodynamic quantities
The thermal effect characterizing the bonding strength of the solute with active sites of the adsorbent was determined by the relationship between the distribution coefficient and the thermodynamic parameters.
The distribution coefficient was calculated by the following equation [3,40]:
Ln Kd=(ΔS?/R)-(ΔH?/RT) (8)
R, ideal gas constant. T, temperature (K). Kd, distribution coefficient (amounts of removed MB per gram of material divided by its concentration in the liquid phase).
The plot of ln(Kd) versus 1000/T gives a straight line, the slope and the intercept correspond to ΔH?/R and ΔS? /R, respectively.
The plot of ln(Kd) versus 1000/T for MB sorption is represented in Figure 10, and the thermodynamic quantities are regrouped in Table 4.
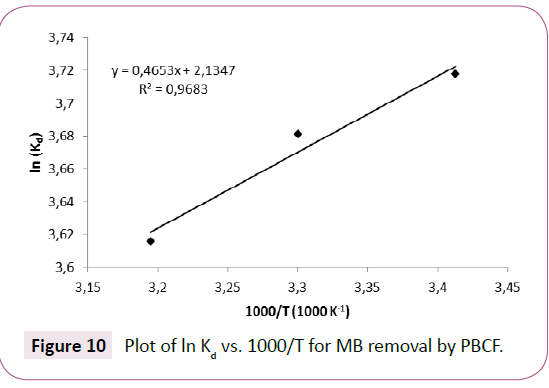
Figure 10: Plot of ln Kd Figure 10 vs. 1000/T for MB removal by PBCF.
| ΔS? (J mol−1 K−1) | ΔH? (kJ mol−1) | ΔG? (kJ mol−1) | ||
| 293°K | 303°K | 313°K | ||
| 17,76 | -3,87 | -9,07 | -9,25 | -9,43 |
Table 4: Thermodynamic parameters for Methylene Blue sorption on PBCF.
Negative value of ΔH?(-3.87 KJ.mol-1) and ΔG? (-9.075 at 293°K, -9.25 at 303°K and -9.43 KJ.mol-1 at 313°K) indicate that the sorption process was exothermic and almost spontaneous respectively. Positive value of ΔS? (17.76 J mol-1 K-1) demonstrate the presence of a disorder at the solid–liquid interface during the sorption of the MB on the substrate [3].
Conclusion
This study showed that the Prickly bark of cactus fruit is considered as a low cost natural sorbent. A contact time of 60 min is quite sufficient for the sorption experiments. The sorption data and kinetic of Methylene blue onto PBCF fitted well the Ho’s pseudosecond- order model with a good determination coefficient. The mathematical Langmuir and Freundlich models showed that the sorption data of Methylene blue onto the prepared bio-sorbent is favorable with a better sorption capacity 222.22 mg g-1), than those of many sorbent cited in the literature. The thermodynamic parameters and experimental data revealed that the sorption was, spontaneous, exothermic and feasible. EDS, FTIR, and iodine number studies indicate the presence of oxygenated functional groups with a high surface area. The percentage of methylene blue fixation increases according the amount of PBCF mass, this explained by the high number of reactive vacant sites the mass transfer and concentration gradient is high.
The highest sorption capacities were recorded at pH greater than pHZPC which gives a negative charge to the bio sorbent surface which leads to the increase of the fixation of the dye positively charged.