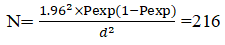Research Article - (2025) Volume 10, Issue 1
Seroprevalence of Brucellosis in Small Ruminants and Public Awareness towards Brucellosis in Two Districts of West Guji Zone, Southern Oromia, Ethiopia
Miessa Banata1*,
Yehualashet Behailu2,
Solomon Mekuria3 and
Kassaye Aragaw3
1Department of Veterinary Public Health, Hawassa University, Hawassa, Sidama, Ethiopia
2Department of Veterinary Epidemiology, Hawassa University, Hawassa, Sidama, Ethiopia
3Department of Veterinary Medicine, College of Natural and Computational Science, Hawassa University, Hawassa, Sidama, Ethiopia
*Correspondence:
Miessa Banata, Department of Veterinary Public Health, Hawassa University, Hawassa, Sidama,
Ethiopia,
Tel: 979850192,
Email:
Received: 09-Jan-2024, Manuscript No. IPJHCC-24-18871 ;
Editor assigned: 11-Jan-2024, Pre QC No. IPJHCC-24-18871 (PQ);
Reviewed: 25-Jan-2024, QC No. IPJHCC-24-18871 ;
Revised: 06-Jan-2025, Manuscript No. IPJHCC-24-18871 (R);
Published:
13-Jan-2025, DOI: 10.36648/2472-1654-10.1.61
Abstract
Brucellosis is one of the most widespread zoonotic illnesses in the world. A close human-animal contact and tradition of raw animal product consumption make zoonosis among the major public health hazards, with particular implication to pastoral area such as West Guji zone. Lack of sufficient awareness regarding the disease in the area is another considerable issue. A cross sectional study was conducted with the objectives of estimating seroprevalence of small ruminants’ brucellosis, assessing associated risk factors and understanding the community awareness towards the disease. Systematic sampling methods were used to select the study animals. Accordingly, 324 small ruminants (132 sheep and 192 goats) and 52 human sera samples were collected. These samples were first screened by Rose Bengal Plate Taste (RBPT) and then positive ones were finally confirmed by I-ELISA. Out of which 34 small ruminants and 13 human samples were positive using RBPT of these 23 ruminants and 10 human samples were confirmed using I-ELISA. An overall seroprevalence of brucellosis was 7.1% and 19.2% in small ruminants and humans respectively. Univariable logistic regression showed that risk factors such as the sex, abortion history, age group, flock size, management, BCS, and parity were significantly associated (p<0.05) with increased seropositivity in small ruminants. Whereas in multi variables logistic regression age group, abortion history, flock size, BCS and parity showed significant difference. Adult age, animal with abortion history, large flock size, poor body condition and multiparous animals were more likely infected than their respective counterparts. Seroprevalence in humans was high in adult, females and those with problem of sanitation. So, these results provided evidence of the importance of brucellosis in humans and small ruminants in the study area. Overall, the existence of brucellosis, the community's daily practice of uncontrolled movement of animals, and the livelihood nature of pastoralists suggest the need for public health education on the zoonotic importance of brucellosis continuously in the study area.
Keywords
Brucellosis; Dugda Dawa; Seroprevalence; Small ruminant; Suro Berguda
Introduction
Background of the Study
One of the most widespread zoonotic illnesses in the world, brucellosis, is diagnosed in over 500,000 people annually. The worldwide illness load on cattle is likewise significant. The Near East, the peninsula of the Balkans, Central Asia, and portions of Africa and South America are all plagued by the disease. The most current reviews of the brucellosis prevalence in humans worldwide were conducted by Khan and Zahoor.
Brucella species are the primary source of the zoonotic bacterial illness known as brucellosis, which mostly affects animals, while humans serving as accidental hosts. Despite being a significant public health issue, the illness is often ignored across the world. It is the second-most significant zoonotic disease next to salmonellosis in the world, according to OIE [1]. The illness is more significant in developing nations and has significant negative effects on the economy and public health. It is an industrial disease that mostly affects butchers, farmers, veterinarians, stock inspectors, employees of abattoirs, and laboratory workers. The World Health Organization (WHO), has designated the illness as one of the world's top "neglected zoonotic diseases" because of the impact it exerts particularly on low-income nations.
The existence of small ruminant brucellosis in sub-Saharan Africa has been confirmed, but the full economic and zoonotic effects of the illness have only been partially studied, if at all. Additionally, almost all of reports have relied on serological data. Due to its vast distribution and effects on several species of animals, especially cattle, sheep, goats, pigs, and people, the disease is one of the highest priority diseases in sub- Saharan Africa and other developing nations.
According to the available serological data, the prevalence has been discovered to vary from location to location based on agro ecology, management, flock size, as well as host-related variables discovered a link between reproductive inefficiencies and Brucella exposure, whereas Asmare et al., revealed that prevalence tends to increase in adults kept in larger flocks. On the other hand, because the etiological agents have not been discovered in Ethiopia, exposure research involving zoonosis could not be linked to any of the known Brucella species.
Bovine, ovine, caprine, swine, and several other domestic animals, including camels, are often affected animals and have an increased risk of abortions in the third trimester of pregnancy due to infection. Animals' chief symptoms of infection include abortions in females and epididymitis and orchitis in males. Only laboratory testing, which may even detect latent infections, may definitively diagnose an infection. The illness has been compared to TB as a result of this as well as the granulomatous form of the lesions.
It is a direct contributor to economic losses due to clinical illness, abortion, neonatal deaths, decreased fertility and reduced milk production. According to Renukaradhya et al., breeding inefficiency, lamb and young loss, and decreased wool, meat, and milk production are the main causes of financial losses in small ruminants. It also plays a significant role as a barrier for international trade of live animals by being used as an impediment to free animal movement and export.
The predilection locations for brucellosis are the male and female reproductive systems, particularly the uterus during pregnancy. It is mainly a disease of sexually active animals. Most Brucella is stimulated to proliferate by allantoic stimuli. These factors include erythritol, possibly steroid hormones and other substances.
The close contact between humans and animals as well as the custom of eating raw animal products make zoonosis one of the biggest risks to public health, with specific implications for pastoral communities. This necessitates a comprehensive epidemiological analysis that takes into account identifying the key risk variables that predominately impact the development of the illness and helps to build effective and workable national control plans.
Depending on the animal species involved, Brucella spp. management techniques, and the accessibility and effectiveness of vaccinations, brucellosis in farm animals can be controlled and prevented. Immunization, testing, removal, and better management methods and movement control are all approaches for controlling the illness. The one health approach to manage and prevent human and animal brucellosis, however, is a crucial strategy for brucellosis control that has gained increasing global attention in recent years.
Materials and Methods
Description of the Study Area
This study was conducted in West Guji zone, which is located in the Southern part of Ethiopia. The capital town of the zone is Bule Hora, which is 467 km away from both the regional and national capital city of Ethiopia (Addis Ababa) [2]. The West Guji zone is bounded by SNNP and Sidama Regional State in the North, the Southern Ethiopia state in the West, the Guji zone in the East, and the Borena zone in the South. Geographically, the zone is located between 37°56' and 38°31' East longitude and 5°26' and 5°52' North latitude, at altitude ranging from 1500 and 2400 m above sea level.
Out of the nine districts that make up West Guji Zone, this research focused on Dugda Dawa and Suro Berguda. According to the zone livestock resource and development Office, the West Guji zone 2,432,796 cattle, 1,230,518 sheep, 1,735,586 goats, 696,230 equines (horse 347,101, donkey 290,422, mule 58,707), and 2,212,928 poultry.
Dugda Dawa: The district is one of the pastoralist areas of the zone located 498 kilometers from Addis Ababa, and 30 kilometers from the zonal town of Bule Hora. The climate consists of 30% mid-altitude and 70% lowland, with an average annual temperature of 25.7-33°C and annual rainfall between 430-500 mm. According to the district livestock resource and development office, there are 118,272 chickens, 195,998 cattle, 6,348 goats, 133,008 sheep, 42,076 equines (38,694 donkeys, 1796 mules, and 1,568 horses), and 6,348 goats in the livestock population.
Suro Berguda: The District lies 495 km south of Addis Ababa. The average yearly temperature ranges from 19.8 °C to 28.7 °C. The average annual rainfall is between 450 and 500 millimeters, with lengthy and brief rainy seasons. The short rainy season lasts from mid-September to mid-November, whereas the long rainy season lasts from March to May. The district is located between 1500 and 2000 meters above sea level. The population of animals consists of 100,214 chickens, 27,694 donkeys, 1696 mules, 3,348 sheep, 112,005 goats, 30,076 horses, and 155,998 cattle (Figure 1).
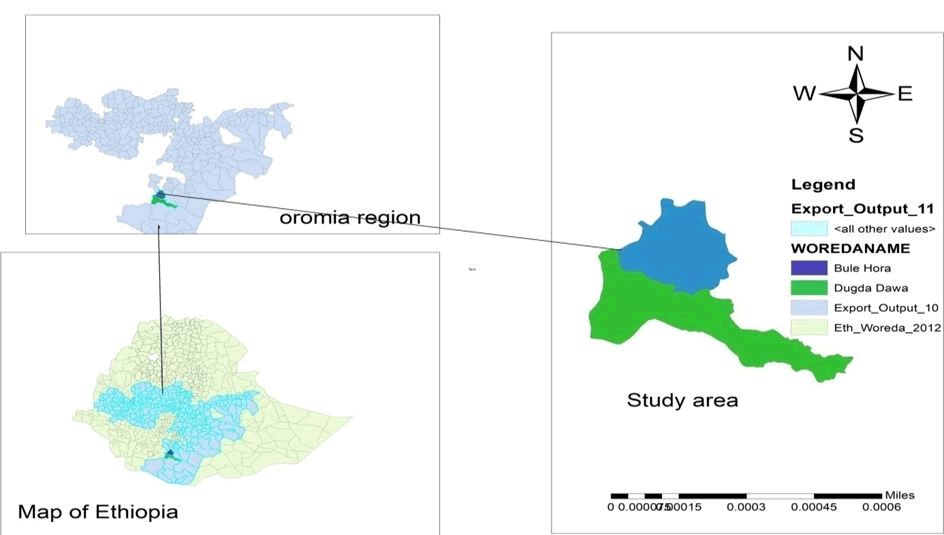
Figure 1: Map of study area.
Study Populations
Sheep and goats that are 6 months of age and older are managed under extensive and semi-extensive pastoral production systems in the Dugda Dawa and Suro Berguda districts of West Guji and were designated as the study population. Samples were taken from human patients who had visited Bule Hora university teaching hospital and had frequent interaction with small ruminants.
Study Design
The study used a cross-sectional design in order to estimate the prevalence of brucellosis in small ruminants and humans in these two districts of West Guji zone, to identify potential risk factors linked to seropositivity, and gauge small ruminant owners' awareness of the disease's significance for public health, the study was carried out in Dugda Dawa and Suro Berguda. Following the collection of a list of the districts in the West Guji zone, two districts (Dugda Dawa and Suro Berguda) were chosen from a total of nine districts based on potential of small ruminant population and their proximity to a local veterinary laboratory. Target Kebeles (Jigesa, Arbicho, and Mokonisa from Dugda Dawa and Danbala Hara and Sororo Malka Jawe from Suro Berguda) in the two districts were chosen based on their small ruminant populations, while communities were chosen more conveniently based on their ease of access by car.
Sampling Method and Sample Size Determination
Sampling method: A combination of random, purposive, and convenient sampling techniques were applied for the selection of study animals (sheep and goats), study areas (district and kebele), and study villages respectively [3]. Thus, the study districts and Kebeles were chosen purposively based on the geographic proximate of the location to the regional veterinary laboratory and small ruminant population; individual sheep and goats were selected using a systematic random sampling technique, and 52 human were chosen at random from the Bule Hora University teaching hospital to assess the public health importance of brucellosis in humans, according to Wubishet et al., the patient based on the presence of symptoms (fever, sweat, anorexia, malaise, weight loss, depression, headache, and joint pains) resembling that of brucellosis, on the history of consumption of unpasteurized milk, raw meat, contact with aborted animals or aborted materials, and handling of parturient animals (those who came from the Dugda Dawa and Suro Berguda districts).
Relevant individual's general information and flock level information such as sex, age flock size presence or absence of reproductive problems such as abortion history were also recorded. Based on literature of Solomon et al., flock size was grouped into three; large, medium, and small based on the number of animals in the flock. So that number of shoats greater than 30 (>30), number of shoats greater than or equal to 10 (≥ 10) but less than thirty (<30), and number of shoats less than or equal to ten (≤ 10); were classified as large, medium and small flock size respectively.
Sample size determination
Small ruminants: Sample size was determined using the formula presented by Thrusfield. Previous report prevalence of 6.1 and 9.2% brucellosis in sheep and goats, respectively reported by Wubishet et al., were used to calculate sample size. Given a target absolute accuracy of 5% and a confidence interval of 95%, the required sample size was determined as:
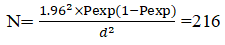
Where, N is the sample size, P is the prevalence, and D is the required degree of accuracy (5%).
Based on the calculation above, the estimated sample sizes for sheep and goats were 88 and 128 respectively. To improve the accuracy of the result, the calculated sample size was increased to 192 goats and 132 sheep. So, 324 shoat in total were chosen for this investigation [4]. Age, sex, parity, and a history of reproductive issues (abortion and retained fetal membrane) were documented for each animal.
Humans: Fifty-two human samples were purposively collected from those patients who had visited Bule Hora university teaching hospital.
Blood Sample Collection
Humans: Prior to collecting samples from humans, participants or their parents or legal guardians were informed of the study's objectives and given the opportunity to agree. Blood sample collection and humans’ samples were both recorded. In humans, verbal consent was made before a sample of 5-7 ml of peripheral blood was taken from each respondent. Blood samples from the human subjects were taken by nurses working at the teaching hospital of Bule Hora University. To facilitate blood clotting, the sample was left to stand on the rack overnight. After that, the sera were poured into sterile cryovials and kept at -20°C until a lab test was done.
Small ruminants: After the animals were appropriately secured, an 8 ml blood sample was aseptically taken using sterile plain vacutainer tubes from the jugular vein. To facilitate blood clotting, the sample was left to stand on the rack for the duration of overnight. After that, the sera were poured into sterile cryovials and kept at -20°C until a lab test was done. During blood collection, owners provided information regarding the size of the flock, the type of the animals, their ages and sexes, the occurrence of abortions, and retain of fetal membranes, which was noted in the sample collection format were registered [5].
Questionnaire Survey
The owners of small ruminants were interviewed using a semi-structured questionnaire about potential risk factors such as management practices, breeding practices, watering practices, age of the animals, sex and educational levels of respondents, awareness of the causes of reproductive disorders, keeping of animals in the dwelling house, and limitations on livestock production. The format, created for this purpose is used to record any detected reproductive abnormalities. The presence of symptoms suggestive of brucellosis in humans (fever, sweat, anorexia, malaise, weight loss, depression, headache, and joint pains), the practice of consuming unpasteurized milk, contact with aborted animals or aborted materials, and handling of parturient animals were all taken into consideration when determining the disease's significance for public health recorded at format forwarded.
Serological Diagnostic Tests
Rose Bengal Plate Test (RBPT): All sheep and goats’ serum samples were initially screened using a Rose Bengal plate test using 75:25 μL, sera: Antigen ratio, whereas, a 30:30 μL ratio (antigen: serum ratio) was used for humans [6-8]. The sera and antigen kept in the refrigerator were taken out from the refrigerator and left at room temperature. Briefly, RBPT antigen of 25 μL was added onto a glass slide next to 75 μL of sheep or goats’ serum. The antigen and the test serum were mixed thoroughly in a plastic applicator, shaken for 4 minutes and then the result was read immediately as described. Any observed agglutination by the naked eyes was considered to be a positive reaction. Agglutination was recorded as 0, +, ++ and +++. A score of 0 indicates the absence of agglutination; + indicates barely visible agglutination; ++ indicates fine agglutination, and +++ indicates coarse clumping. Those samples with no agglutination (0) were recorded as negative while others were recorded as positive.
Indirect Enzyme-Linked Immuno-Sorbent Assay (I-ELISA): Subsequently, the positive reactors to RBPT were reinvestigated using Indirect Enzyme-Linked Immuno-Sorbent Assay (I-ELISA) for further confirmation and used to detect antibodies against Brucella at Yabello regional veterinary laboratory for small ruminants and simultaneously human sera process in Bule Hora University teaching hospital. The taste was conducted in a micro-plate coated with activated antigens following manufacturer’s instructions. One hundred micro litters of pre-diluted sera and controls (1:400) were added in to the micro-titer plate and incubated for 45 min at 21°C. The plate was then washed 3 times. Then 100 μL of conjugate was added to each well followed by covering of the plates before incubating it for 30 min at 21°C. The plates were washed 3 times. Finally, 100 μL of substrate was added to each and incubated at 21°C for 15 min. Then 100 μL of stop solution was added to each well and the result was read at a wavelength of 450 nm. Results were expressed as the percentage of the ratio between the corrected sample OD and positive control OD. The taste conducted according to the manufacturer guidelines [9-11].
Data Management and Analysis
The collected data were entered into Microsoft Excel® 2010 spread sheet; variables that are hypothesized to be connected to the epidemiology of brucellosis in humans and small ruminants were noted. Animal owners and attendants provided information on issues with reproduction, animal age, and parity numbers. Data on a person's age, gender, how they consume animal products, how they handle aborted fetuses, and how they help animals during parturition were also recorded. Microsoft excel was used to capture and code the data, and STATA version 14 was used to conduct the analysis. The Odds Ratio (OR), which represents the degree of a risk factor's association with a 95% confidence interval, was used to identify risk variables linked to Brucella infection using a multivariable logistic regression model. In the analyses, 95% confidence intervals were generated, and a level of statistical significance of (P<0.05) was applied.
Ethical clearance: Ethical clearance for animal sampling was obtained from the Hawassa University faculty of veterinary medicine and ethical clearance for humans was approved by the research ethics committee and the letter of clearance was obtained from the Hawassa University ethical committee. The sample was taken after written informed consent was made with all study participants. All the rights of privacy and confidentiality of participants are protected [12].
Results
Seroprevalence of Brucellosis in Small Ruminants
Out of 324 blood samples collected from sheep and goat populations in Dugda Dawa and Suro Berguda districts (192 caprine and 132 ovine); 34 (10.49%) tested positive by RBPT. Further confirmatory test conducted for positive reactors using I-ELISA, of these 23 samples (animals) were positive for Brucella infection (7.1%). Out of 147 tested sera in Dugda Dawa district, 15 was positive by I-ELISA (10.2%) and out of 177 tested in Suro Berguda district, 8 (4.5%) were positive by I-ELISA, as shown in (Figure 2).
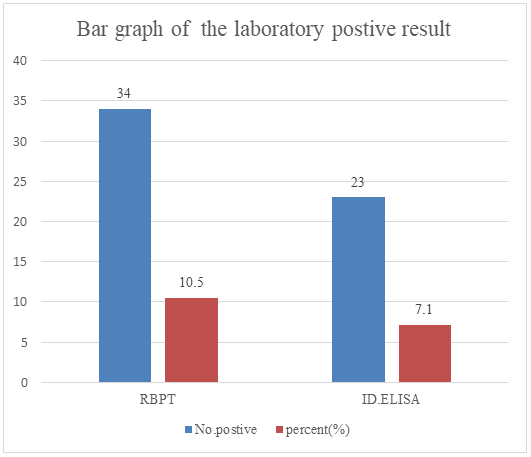
Figure 2: The overall seroprevalence of small ruminants’ brucellosis.
Risk Factors Affecting Individual Animal Level Seroprevalence of Brucellosis
Logistic regression showing seroprevalence of small ruminants’ brucellosis with associated risk factors including; district, peasant association, species, age, flock size, management, history of abortion, body condition score, and parity numbers showed in Table 1. The individual animal level seroprevalence was higher in Dugda Dawa 15 (10.2%) than in Suro Berguda 8 (4.5%) and goats (8.85) than in sheep (4.54%) populations, but this result was not statistically significant (P>0.05). The seroprevalence in the present study, in Table 1 shown that, the prevalence higher in females’ animals (9.3%) than in males (2.75%), elders’ animals (17.6%) than youngers (2.25%), larger flock size (26.6%) than smaller (1.37%), extensive management system (11.3%) than semi-extensive (3.4%), animals aborted (37.7%) greater than not aborted (2.2%), poor BCS (18.9%) greater than good BCS (2.4%) and animal with multiple parturition (25.5%) greater than non-parous (1.7%).
| Variables |
Categories |
No. of sampled |
Prevalence (%) |
Odds Ratio |
95% CI |
P>|z| |
| Districts |
D. Dawa |
177 |
15 (8.5) |
Ref |
Ref |
Ref |
| S. Barguda |
147 |
8 (5.4) |
2.4 |
0.99-5.83 |
0.05 |
| Pa |
Jigesa |
79 |
7 (8.8) |
Ref |
Ref |
Ref |
| Arbicho |
67 |
7 (10.4) |
1.24 |
0.40-3.76 |
0.71 |
| Mokonisa |
31 |
1 (2.7) |
2.26 |
0.26-19.6 |
0.45 |
| D. Hara |
69 |
4 (5.7) |
2.26 |
0.63-8.06 |
0.21 |
| S/M/Jawe |
78 |
4 (5.1) |
2.91 |
0.70-8.92 |
0.16 |
| Spp |
Ovine |
132 |
6 (4.54) |
Ref |
Ref |
Ref |
| Caprine |
192 |
17 (8.85) |
2 |
0.78-5.31 |
0.16 |
| Sex |
Male |
109 |
3 (2.75) |
Ref |
Ref |
Ref |
| Female |
215 |
20 (9.3) |
3.6 |
1.05-12.47 |
0.04 |
| Age |
Young |
222 |
5 (2.25) |
Ref |
Ref |
Ref |
| Adult |
102 |
18 (17.6) |
9.3 |
3.4-25.9 |
0 |
| Flock size |
Small |
145 |
2 (1.37) |
Ref |
Ref |
Ref |
| Medium |
119 |
5 (4.2) |
3.13 |
0.59-16.4 |
0.17 |
| Large |
60 |
16 (26.6) |
26 |
5.75-11.49 |
0 |
| Management |
Se_extensive |
174 |
6 (3.44) |
Ref |
Ref |
Ref |
| Extensive |
150 |
17 (11.33) |
3.6 |
1.41-9.33 |
0 |
| Abortion history |
No |
279 |
6 (2.2) |
Ref |
Ref |
Ref |
| Yes |
45 |
17 (37.7) |
27.6 |
10.07-75.7 |
0 |
| Poor |
95 |
18 (18.94) |
Ref |
Ref |
Ref |
| BCS |
Medium |
146 |
3 (2.05) |
11.2 |
3.91-40.0 |
0 |
| Good |
83 |
2 (2.40) |
9.5 |
2.12-44 |
0 |
| None |
229 |
4 (1.74) |
Ref |
Ref |
Ref |
| Parity |
1-3 |
48 |
7 (14.58) |
9.6 |
2.6-34.2 |
0 |
| Above 3 |
47 |
12 (25.53) |
19.2 |
5.88-63.1 |
0 |
Table 1: Univariable logistic regression analysis of small ruminant brucellosis prevalence relative to different factors by I-ELISA.
Multivariable logistic regression analysis of risk factors for brucellosis reactivity: The risk factors with significant effects after the univariate logistic regression test (sex, age group, flock size, management, abortion history, BCS, and parity) were fitted in a multivariate model. The results revealed that among the risk factors considered in the analysis, age, flock size, management, abortion history and BCS had statistically significant effects on seropositivity (p<0.05). The outcome of a multivariable logistic regression analysis of risk factors brucellosis reactivity in the research region is shown in Table 2.
| Variables |
Categories |
No. of sampled |
Prevalence (%) |
Odds ratio |
95% CI |
P>|z| |
| Sex |
Male |
109 |
3 (2.75) |
Ref |
Ref |
Ref |
| Female |
215 |
20 (9.3) |
1.08 |
0.23-5.04 |
0.92 |
| Age |
Young |
222 |
5 (2.25) |
Ref |
Ref |
Ref |
| Adult |
102 |
18 (17.6) |
10.3 |
2.92-36.7 |
0 |
| Flock size |
Small |
145 |
2 (1.37) |
Ref |
Ref |
Ref |
| Medium |
119 |
5 (4.2) |
3.2 |
0.59-17.3 |
0.17 |
| Large |
60 |
16 (26.6) |
29.5 |
6.18-141.6 |
0 |
| Management |
Se_extensive |
174 |
6 (3.44) |
Ref |
Ref |
Ref |
| Extensive |
150 |
17 (11.33) |
3.4 |
1.06-11.3 |
0.04 |
| Abortion history |
No |
279 |
6 (2.2) |
Ref |
Ref |
Ref |
| Yes |
45 |
17 (37.7) |
10.3 |
2.40-44.5 |
0 |
| Poor |
95 |
18 (18.94) |
Ref |
Ref |
Ref |
| BCS |
Medium |
146 |
3 (2.05) |
12.6 |
3.06-53.2 |
0 |
| Good |
83 |
2 (2.40) |
5.3 |
0.98-28.5 |
0.05 |
| None |
229 |
4 (1.74) |
Ref |
Ref |
Ref |
| Parity |
1-3 |
48 |
7 (14.58) |
2.3 |
0.39-13.2 |
0.35 |
| Above 3 |
47 |
12 (25.53) |
4.74 |
0.88-25.5 |
0.06 |
Table 2: Multivariable logistic regression analysis of small ruminant brucellosis prevalence relative to different factors by I-ELISA.
Serological results of human brucellosis: As shown below in Table 3, out of 52 serum samples of humans tested for RBPT only 13 were positives for agglutination test. 4 (16.6) males and 9 (27.2) females were, Analysis of laboratory results in human sera indicates that human sera tested for I-ELISA 3 (12.5) males and 7 (21.2) females positive for this serological taste [13]. The overall sero-prevalence of human brucellosis among the blood samples tested was 19.2% in this study. The positivity between males and females was observed (OR=3.43, 95% CI: 0.77-15.1). The highest seroprevalence was recorded in individuals those no washed their hands with soap after contact with animals (29%) than those washed their hands (8%) and was statistically significant (p<0.05) (Figure 3).
| Variables |
Categories |
No. of sampled |
Prevalence (%) |
Odds ratio |
95% CI |
P>|z| |
| Districts |
Dugda Dawa |
28 |
5 (17.85) |
Ref |
 Ref |
Ref |
| Suro Berguda |
24 |
5 (20.83) |
1.21 |
0.30-4.8 |
0.78 |
| Jigesa |
11 |
1 (9.0) |
 Ref |
Ref |
Ref |
| Pa |
Arbicho |
8 |
2 (25) |
3.33 |
0.24-45 |
0.36 |
| Mokonisa |
9 |
2 (22) |
2.85 |
0.21-38 |
0.42 |
| Danbala Hara |
11 |
2 (18.2) |
2.22 |
0.17-28 |
0.54 |
| Soror M/Jawe |
13 |
3 (23) |
3 |
0.26-34 |
0.37 |
| Sex |
Male |
24 |
3 (12.5) |
|
Ref |
Ref |
| female |
33 |
7 (21.2) |
3.43 |
0.77-15 |
0.1 |
| Age |
Adult |
33 |
9 (27.9) |
|
Ref |
Ref |
| Young |
19 |
1 (5.26) |
7.4 |
0.86-67 |
0.06 |
| Help delivery |
Yes |
37 |
8 (21.6) |
|
Ref |
Ref |
| No |
15 |
2 (13.3) |
1.79 |
0.33-10 |
0.49 |
| Consume raw meat |
Yes |
40 |
8 (20) |
|
Ref |
Ref |
| Consume raw milk |
 No |
12 |
2 (18) |
1.25 |
0.23-6.9 |
0.79 |
| Â Yes |
39 |
8 (20.5) |
|
Ref |
Ref |
| Contact manure |
 No |
13 |
2 (15.3) |
1.41 |
0.26-7 |
0.68 |
| Yes |
37 |
7 (18.9) |
|
Ref |
Ref |
| Wash hands with soap |
No |
15 |
3 (20) |
1.2 |
0.26-5.5 |
0.8 |
| Yes |
25 |
2 (8) |
|
Ref |
Ref |
| No |
27 |
8 (29) |
5.8 |
1.11-31 |
0.03 |
Table 3: Association of risk factors with brucellosis reactivity in humans.
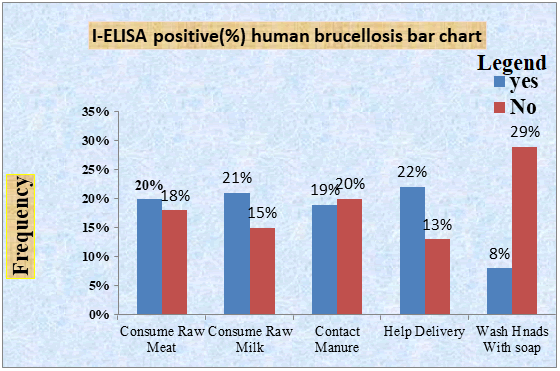
Figure 3: Laboratory result of human brucellosis using I-ELISA.
Results of Questionnaire Survey
Interviewer administered face to face questionnaire was used to evaluate the knowledge and practice of the community on brucellosis. The questionnaire was pretested in the field.
Finally, it was translated to local language Afan Oromo. As indicated in Table 4, a total of 50 proprietors of small ruminants from two districts and five Kebeles received the questionnaire [14]. The findings showed that just 11 (22%) of these individuals had separate house for your sheep and goats, while the remaining 39 (78%) had no separate house for your sheep and goats. 46 (92%) of the pastoralists surveyed consume raw milk. Similarly, 37 (74%) of respondents were unaware that consuming raw milk may transmit brucellosis. The majority of 41 volunteers (82%) aided parturition without safety precautions. Regarding methods of handling and treating aborted fetuses and placentas, 29 (58%) left the placenta on the ground, while 21 (42%) fed it to dogs. In response to the question of what they did with their animals when a they abort, 14 (28%) said they sold them, 20 (40%) said they treated animals themselves, and only 16 (32%) said that they brought the afflicted animal to a local veterinary clinic.
| Variables |
Categories |
Numbers |
Percentage |
| Do you use separate house for your sheep and goats? |
Yes |
11 |
22 |
| No |
39 |
78 |
| Do you know diseases which cause abortion? |
Yes |
18 |
36 |
| No |
32 |
64 |
| Do you separate aborted animal from others? |
Yes |
50 |
100 |
| No |
0 |
0 |
| Do you assist lambing or kidding? |
Yes |
50 |
100 |
| No |
0 |
0 |
| Do you assist by your bare hand? |
Yes |
41 |
82 |
| No |
9 |
18 |
| Do you wash your hands with soap and water after kidding or lambing? |
Yes |
23 |
46 |
| No |
27 |
54 |
| Means of meat consumption |
Raw |
46 |
92 |
| Cooked |
4 |
8 |
| Means of milk consumption |
Raw |
37 |
74 |
| Fermented |
13 |
26 |
| Boiled |
0 |
0 |
| Feed to dog |
21 |
42 |
| Means of managing aborted foetus/FM |
Bury/Burn |
0 |
0 |
| Leave on ground |
29 |
58 |
| Purpose of animal when aborted |
Treated by their own |
20 |
40 |
| Take to vet. clinic |
16 |
32 |
| Sell |
14 |
28 |
| Existence of abortion and retained foetal membrane in your flock |
Yes |
50 |
100 |
| No |
0 |
0 |
Table 4: Awareness of small ruminant owners about brucellosis.
Discussion
In the study districts, the overall prevalence of small ruminants was 7.1%, while the prevalence at the species level remained at 4.54% for sheep and 8.85% for goats. This result is generally in line with the findings of Wubishet et al., and Ashenafi et al., from the Borena zone in the Oromia region and Afar region, respectively; and they reported an overall prevalence of 8.1%, with species level prevalence of 9.2% and 6.1% in goat and sheep in Borena zone and overall prevalence of 4.8%, species level prevalence of 5.8% in goats and 3.2% in sheep from Afar region, respectively.
The 7.1% overall seroprevalence found in the current study was significantly higher than the overall seroprevalence previously reported, which was 1.6% by Debassa et al., 3.3% by Sintayehu et al., from the same agro ecology of Borena pastoralist area, 1.6% by Mengistu from Konso, Southern Ethiopia, and 3.5 by Teklue et al., from Southern Tigray. Furthermore, Teshale et al., from the Somali pastoral region reported 1.7% in goats and 1.6% in sheep, while Bekele et al., from the same region reported 1.9% in goats and 1.2% in sheep, both of which showed far lower species level seroprevalence than the current study. Additionally, the present finding was higher than the results from certain African nations. Benkirane from Eritrea measured 4.1% in goats and 1.6% in sheep. Animal level prevalence was also found to be 15% in sheep and 16.5% in goats from the Afar area by Yibeltal [15]. The observed difference in prevalence could be due to the variation in sensitivity and specificity of the various tests, sample size of the study.
Although it was not statistically, significant (p>0.05), difference in seropositivity among sheep and goats was found in the current investigation, with goats, recording a higher seropositivity of 8.85% than sheep 4.54%. This situation matched the findings of earlier research by Ashenafi et al., which found 3.2% and 5.8% in sheep and goats from the Afar area of northern Ethiopia, respectively. These findings are also consistent with that of Aworh et al., who reported prevalence of 19.6% and 9.4% in caprine and ovine, respectively, from animals butchered at abattoirs in Abuja, Nigeria. They found that caprine had a higher seroprevalence of brucellosis than ovine. Compared to sheep, goats are more likely to get Brucella infection. This could be a result of goats being more susceptible to Brucella infection. It could possibly be partially because goats, as opposed to sheep, expel the organism over a longer period of time. As a result, there is a lower chance of disease transmission within sheep herds.
In the current study, flock level seroprevalence was recorded at 26.6%, which was lower than the results of Wesinew et al., in Afar (50.51%), higher than the results of Edao et al., who recorded flock level prevalence at 22.7% from a Borena pastoralist in Southern Ethiopia, and comparable to Teklu et al., who reported 28.3% from the Southern zone of Tigray region in Northern Ethiopia [16]. In the current investigation, an increase in flock size was directly correlated with an increase in animal seropositivity, and this relationship was statistically significant (p<0.05) when compared to a decrease in flock size. Teklu et al., further validated the phenomena of larger flock size being more susceptible to Brucella infection, finding a significant (p<0.05) association between higher flock size and Brucella seropositivity in small ruminants. According to Radostits et al., herd size and animal density are closely correlated with illness prevalence and the difficulties of controlling infection in the population.
Brucellosis seroprevalence varied depending on the age group of the investigated animals. This finding provided evidence for a highly significant (p<0.05) correlation between Brucella seropositivity and the age of sheep and goats. According to the study, adult brucellosis rates in these settings were higher than those for young (2.25%), at 17.6%. The reports of Megersa et al., from the Borena pastoral region of Southern Ethiopia were generally consistent with the findings of this study. In 2007, Ashenafi et al. from the pastoral Afar area and Ashagirie et al., from the South Omo zone in Southern Ethiopia. Adugna et al., from Afar region, North East Ethiopia. Muma et al., found that being older increases the likelihood of contracting the Brucella bacteria. This may be explained by the fact that only adult, sexually mature males and females contract the disease brought on by infection [17]. Due to the influence of sex hormones and placental erythritol on the pathogenesis of brucellosis, which stimulates the growth and multiplication of Brucella, susceptibility does, however, increase with sexual maturity and pregnancy. However, it is also true that young animals have a higher level of infection resistance and typically recover from an existing illness, even though latent infections can happen.
Male and female small ruminants varied statistically significantly regarding their seropositivity to Brucella. In the current study, female ovines or caprines had a greater seroprevalence of brucellosis (9.3%) than male ovines or caprines (2.75%). This result is consistent with those reported. Moti et al., found that brucellosis affected 3.2 and 1.2% of females and males, respectively, in Southern Ethiopia. A high quantity of erythritol, which is seldom formed in male reproductive organs, may be the cause of the high frequency of brucellosis in females. Due to the formation of erythritol, a 4-carbon sugar in fetal and female reproductive tissues that promotes the growth of Brucella organisms, these tissues have a special affinity for Brucella species, which is why female animals have different levels of Brucella antibodies. Additionally, female animals are often kept in the flock for a longer amount of time than males. Female farm animals live longer time than males do which increases their exposure to Brucella germs and increases their risk of infection.
It was discovered that the prevalence of brucellosis was also higher in animals with a history of abortions and a previous history of retained fetal membrane (37.7%) and lower than the report by Wubishet who reported 50.0% in Yabello district of Borena Zone. Increased as well, and the difference that was seen was statistically significant (p<0.05). Animals that are pregnant were more likely to contract the organism than animals that are still developing sexually. As the gestational stage advances, susceptibility also rises. This suggests that retained placenta and abortions or stillbirths are typical clinical sign of brucellosis. It is a result of Brucella species' affinity for certain important target cells known as trophoblast. During the last stages of ruminant pregnancy, high concentrations of steroid hormones and erythritol appear to work synergistically to promote Brucella growth inside the trophoblast. The ability of trophoblasts to multiply quickly and widely can undermine the placenta's integrity and infect the fetus, leading to abortion or the birth of undeveloped children [18].
The correlation between animal body condition ratings and the prevalence of brucellosis was once again statistically significant (p<0.05). According to the study, animals with poor body condition were more likely (18.94%) to contract brucellosis under these conditions than those with good body condition (2.40%). Tsegay et al., from Ethiopia reported that there was a significant association (p<0.05) between Brucella seropositivity and body condition scores in the study conducted on small ruminants slaughtered at Debrezeit and Modjo export abattoirs that thin animals were more susceptible to Brucella infection than animals of medium and good body conditions. The reason for higher seropositivity observed in sheep and goats with good body condition scores in the current study could be that animals that were walked in search of water and pasture were more likely to come in contact with other infected flocks and therefore remain exposed. Animals that were not going to search for food or those grazed poor pastures around the homesteads came with a penalty in the form of malnutrition which may result in loss of body condition and this then reduced the risk of exposure likely to result from comingling with other infected animals. Nutrition plays a great role in immunity against various infectious diseases. Underfed animals are expected to have a decreased immunity that is manifested by poor body condition.
Human brucellosis seroprevalence in the current investigation was 19.2% by I-ELISA. The study's recorded result is less than the result of 25.6% that was reported in the Borena Zone's Yabello and Dire Districts, 34.1% and 29.4% in Borena and Hamer pastoral area of southern Ethiopia respectively and 16.5% in Chifra District, of Afar region, Ethiopia.
Because the prior study was done on pastoralists with febrile illnesses, it is possible that the current study's lower prevalence compared to the previous studies is due to changes in the study population [19]. The current study's findings are higher than those of the majority of highland investigations. Higher incidence can be attributed to differences in the agroecology and local customs (handling aborted fetuses and fetal membranes, consuming raw milk and blood). These practices are thought to be the primary means of disease transfer from animals to humans. To, prevent infection, one should avoid consuming raw milk and blood, handling aborted fetuses or retained fetal membranes with one's bare hands, leaving or putting aborted materials in the environment, and touching vaginal secretions. This survey's findings are consistent with the research that has been published by Kassahun et al., Mussie et al., and Asmare et al.
According to studies by Ragassa et al., Tolosa et al, and Asmare et al., there are likely many unrecognized cases of febrile diseases, osteoarticular complications (joint problems), and other generalized complications in pastoral communities. Characterizing the bacterial species present in humans and small ruminants is beneficial, but I was unable to do this test owing to budgetary constraints and a lack of a nearby, wellequipped laboratory for the diagnosis and investigation of zoonotic diseases.
The current study's findings regarding the practice of handling aborted fetuses and aborted materials (placenta) with bare hands and drinking raw milk were consistent with findings from a related study area reported by Wubishet et al., who found that 120 (95.3%) pastoralists drank raw milk and handled aborted fetuses with bare hands. Similarly, Tegegn et al., stated a similar scenario with the study conducted on sheep and goats raised in urban, peri-urban, and rural regions of Niger overall, as stated by Boukary et al., revealed a similar scenario [20]. The current findings showed that livestock owners in the studied areas were at significant risk of catching brucellosis from diseased animals. According to several experts, the consumption of raw livestock products and the fact that people become sick due to a lack of community knowledge about brucellosis might both contribute to the disease's continued spread.
Conclusion
The seroprevalence study conducted in this study in the West Guji zone, southern Oromia, Ethiopia's Dugda Dawa, and Suro Berguda Woredas, suggested that brucellosis could be one of the major diseases in areas where people and animals have close contact. In small ruminants, large flock size, poor body condition, female animals with a history of the retained fetal membrane and history of abortion are prone to be seropositive relative to other groups of animals. In this study, most individuals had close contact with small ruminants and known practices that put an individual at risk of brucellosis were common. This is in conformation with the general living standard and cultural conditions in Ethiopia that inherently predispose individuals to zoonotic diseases. In addition, animal husbandry is very traditional in Ethiopia and people most often live with their animals under the same roof, and health-care support for their animals is minimal. These factors were believed to support the spread of the disease between animals as well as from animal to humans in the study area. Increasing age, increasing flock level, poor management practices, and letting aborted material in the environment were also associated with higher prevalence. Fit to support this. The prevalence of brucellosis in humans is greater than that in animals. Age and history of assisting animals’ parturition were factors in the probability of brucellosis seropositivity in people.
Recommendation
Considering the above conclusion into account the following recommendations were forwarded:
- Health education about the mode of transmission of brucellosis and about the disease should be provided to the community regularly.
- Integration of different sectors should be focused on to take action against the community’s exposure to brucellosis.
- Further research on the isolation and characterization of circulating Brucella species in small ruminants and humans should be conducted in the study area to propose effective control measures.
Conflict of Interest
The authors to declare that there is no conflict of interest.
References
- Adugna KE, Agga GE, Zewde GJ (2013) Seroepidemiological survey of bovine brucellosis in cattle under a traditional production system in western Ethiopia. Rev Sci Tech. 32(3):765-763.
[Crossref] [Google Scholar] [PubMed]
- Asmare K, Krontveit RI, Ayelet G, Sibhat B, Godfroid J, et al. (2014) Meta-analysis of Brucella seroprevalence in dairy cattle of Ethiopia. Trop Anim Health Prod. 46:1341-1350.
[Crossref] [Google Scholar] [PubMed]
- Asmare K, Prasad S, Asfaw Y, Gelaye E, Ayelet G, et al. (2007) Seroprevalence of brucellosis in cattle and in high risk animal health professionals in Sidama Zone, Southern Ethiopia. Ethiop Vet J. 11(2):59-68.
- Ashenafi F, Teshale S, Ejeta G, Fikru R, Laikemariam Y (2007) Distribution of brucellosis among small ruminants in the pastoral region of Afar, Eastern Ethiopia. Rev Sci Tech. 26(3):731.
[Crossref] [Google Scholar] [PubMed]
- Ashagrie T, Deneke Y, Tolosa T (2011) Seroprevalence of caprine brucellosis and associated risk factors in South Omo Zone of Southern Ethiopia. Afr J Microbiol Res. 5(13):1682-1476.
[Google Scholar]
- Aworh MK, Okolocha EC, Awosanya EJ, Fasina FO (2017) Sero-prevalence and intrinsic factors associated with Brucella infection in food animals slaughtered at abattoirs in Abuja, Nigeria. BMC Res Notes. 10:1-7.
[Crossref] [Google Scholar] [PubMed]
- Benkirane A (2006) Ovine and caprine brucellosis: World distribution and control/eradication strategies in West Asia/North Africa region. Small Ruminant Res. 62(1-2):19-25.
[Google Scholar]
- Berehe G, Belihu K, Asfaw Y (2007) Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia. Int J Appl Res Vet Med. 5:65-71.
- Bekele M, Mohammed H, Tefera M, Tolosa T (2011) Small ruminant brucellosis and community perception in Jijiga district, Somali Regional State, Eastern Ethiopia. Trop Anim Health Prod. 43:893-898.
[Crossref] [Google Scholar] [PubMed]
- Boukary AR, Saegerman C, Abatih E, Fretin D, Alambedji Bada R, et al. (2013) Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of Niger. PLoS One. 8(12):e83175.
[Crossref] [Google Scholar] [PubMed]
- Coelho AM, Coelho AC, Roboredo M, Rodrigues J (2007) A case–control study of risk factors for brucellosis seropositivity in Portuguese small ruminants herds. Prev Vet Med. 82(3-4):291-301.
[Crossref] [Google Scholar] [PubMed]
- Corbel MJ (2006) Brucellosis in humans and animals. World Health Organization.
[Google Scholar]
- Addis M (2013) Small ruminant brucellosis: Serological survey in Yabello District, Ethiopia. Asian J Anim Sci. 7(1):14-21.
[Google Scholar]
- Edao BM, Ameni G, Assefa Z, Berg S, Whatmore AM, et al. (2020) Brucellosis in ruminants and pastoralists in Borena, Southern Ethiopia. PLoS Negl Trop Dis. 14(7):e0008461.
[Crossref] [Google Scholar] [PubMed]
- Edao BM, Ameni G, Berg S, Tekle M, Whatmore AM, et al. (2023) Whole genome sequencing of Ethiopian Brucella abortus isolates expands the known diversity of an early branching sub-Saharan African lineage. Front Microbiol. 14:1128966.
[Crossref] [Google Scholar] [PubMed]
- Ahmed EY, Ali A, Mesfin A, Deressa A, Girmaye T (2008) Brucellosis as a zoonosis in Chifra district, Afar regional state, Ethiopia.
[Google Scholar]
- European Commission (2001) Brucellosis in Sheep and Goat (Brucella melitensis). In: Report of the scientific committee on animal health and animal welfare of the European Commission. 432:1-44.
- Franc KA, Krecek RC, Hasler BN, Arenas-Gamboa AM (2018) Brucellosis remains a neglected disease in the developing world: A call for interdisciplinary action. BMC Public Health. 18:1-9.
[Crossref] [Google Scholar] [PubMed]
- Genene Regassa GR, Desalew Mekonnen DM, Yamuah L, Hiwot Tilahun HT, Teshome Guta TG, et al. (2008) Human brucellosis in traditional pastoral communities in Ethiopia. Intern J TropMed. 4(2): 59-64.
- Mekonnen H, Kalayou S, Kyule M (2010) Serological survey of bovine brucellosis in barka and arado breeds (Bos indicus) of Western Tigray, Ethiopia. Prev Vet Med. 94(1-2):28-35.
[Crossref] [Google Scholar] [PubMed]
Citation: Banata M, Behailu Y, Mekuria S, Aragaw K (2025) Seroprevalence of Brucellosis in Small Ruminants and Public
Awareness towards Brucellosis in Two Districts of West Guji Zone, Southern Oromia, Ethiopia. J Healthc Commun. 10:61.
Copyright: © 2025 Banata M, et al. This is an open-access article distributed under the terms of the Creative Commons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author
and source are credited.