Keywords
Eggs of sea urchin; Developing embryos; Repair enzyme activities; The
stable soluble protein complexes
Abbreviations
AP: Apurinic/Apyrimidinic; BER: Base Excision Repair (Reversal Base
Restoration); CK: Creatine Kinase; EDTA: Ethylenediaminetetraacetic Acid; kDa:
Kilo Daltons; MM: Molecular Mass; PAGE: Polyacrylamide Gel Electrophoresis;
SPCs: Stable Protein Complexes from the Placenta, Milk, and Urchin Eggs; NMP:
Nucleoside Monophosphate; Rhz: Sphingolipid Rhizochalin; SDS: Sodium Dodecyl
Sulfate; StAP: Alkaline Phosphatase of S. intermedius
Introduction
The study of the molecular mechanisms of the most important
biochemical processes in humans is sometimes not possible
without the use of animal models, including marine creatures.
Analysis of the data obtained from sea-animal and human models
can complement and enhance studies on general molecular
mechanisms of many components including proteins and their
complexes functioning.
This review describes all available published data on different enzymes
and proteins of the sea urchins and the results of a comparison
study on various proteins, peptides, and their complexes
from sea urchins and humans.
Back in the last century, important vantages of sea urchins as an object of biological research were disclosed, namely, the potential
of getting large batches of gametes and synchronously upcoming
embryos, the simplicity in their incubation using controlled
conditions, as well as the ease of surveillance and processing of
the obtained data. Sea urchins present one of the most prevalent
and important squads of invertebrates in the orarian zones
of the World Ocean that have a momentous role in maritime
ecosystems [1-3]. Their eggs and spermatozoa are well known as
an exceptionally convenient model for studying the patterns of
development from embryo to the body, which is very difficult to
achieve in humans. In this review, we analyze data on the investigation
of some enzymes, proteins, and previously undescribed
soluble multiprotein complexes of the sea urchin Strongylocentrotus
intermedius eggs and spermatozoa. We also discuss the
published data related to proteins and enzymes of sea urchins.
Literature Review
Analysis of dynamics of enzymes during
embryonic development
Genomes of all living organisms exist in a dynamic balance between
various continuous DNA damages and removal of the damage
due to an operation of known DNA repair enzymes [4]. The
diminution in DNA repair ability is eventually manifested in different
forms of mutagenesis, carcinogenesis, or cell death, which
are associated with a number of human and other organisms' pathologies.
DNA repair is critical for quickly proliferating cells. DNA
damages inhibit replication progress and can be transformed into
mutations during replication of decisively differentiated cells that
occasionally need to maintain their genome integrity throughout
a body's life. In most organisms, several pathways have been
identified, including forwarding reversal base restoration (BER),
nucleotide resection, mismatch repair, non-homologous final
coupling, and recombination restoration [4]. BER, the most common
type of removal of small non-bulky DNA lesions, which have
great importance to multicellular organisms, refereed from the
embryonic lethality of knockouts destroying the entire path [5].
During the BER, several DNA glycosylases excise damaged bases
from DNA, and then apurinic/apyrimidinic (AP) endonuclease splits DNA at the AP site forming free 3′-OH groups, which are
then used by DNA polymerase for including normal deoxynucleotides.
Finally, DNA ligase recovers the initially damaged
strands [4].
In actively proliferating cells, resembling the cells of developing
embryos, DNA repair is key to the preclusion of the cumulation
of mutations and timing of cell division. Since sea urchins are
synchronously developing embryos, they can be used in large
quantities in different experiments. Thus, they represent a very
commodious model for studying the dynamics of changes in the
functioning of the repair enzymes during their development. Revealing
of embryonic substances, including repair enzymes, proteins,
as well as their complexes, is momentous for understanding
embryo development and their function in all organisms,
including humans.
Samples of early embryos of the sea urchin Strongylocentrotus
intermedius (12 of 26 stages of development) were collected and
lysed according to [6-8]. All 26 stages of the sea urchin development
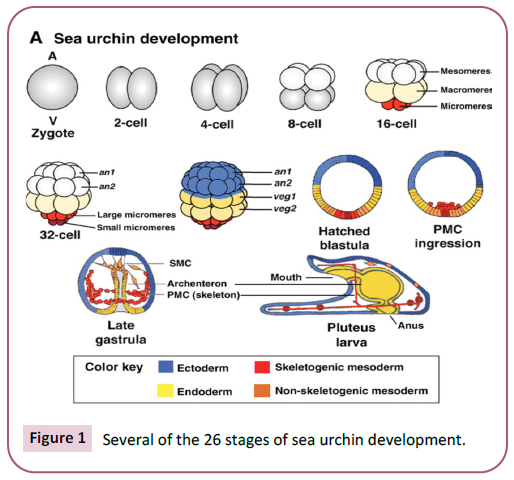
are given in Table 1 [9], and some of them are shown in Figure 1 [9].
| Embryo collection time, hr: min post-fertilization |
Stage number* |
Stages |
30 seconds
0:20 |
1* |
1a. Fertilized egg: formed fertilization shell
1b. Fertilized egg: formed gealine layer |
| 0:30 0:50 |
2 |
2a. The stage of a narrow "strip" (prophase of the first division-cleavage)
2b. The stage of a wide and dumbbell-shaped strip (metaphase - telophase of the first division |
| 1:07 |
3 |
Cleavage (2 blastomeres) |
| 1:45 |
4 |
Cleavage (4 blastomeres) |
| 2:23 |
5 |
Cleavage (8 blastomeres) |
| 3:00 |
6 |
Cleavage (16 blastomeres) |
| 3:38 |
7 |
Cleavage (32 blastomeres) |
| 4:20 |
8 |
Early blastula 1. Blastomeres retain their rounded shape |
| 5:13 |
9 |
Early blastula 2. Blastomeres partially lose their rounded shape |
| 7:15 |
10 |
Middle blastula 1. Blastomeres have lost their round shape |
| 9:30 |
11 |
Middle blastula 2. Hatching - the embryos retain their spherical shape |
| 11:00 |
12 |
Late blastula 1. Single cells of primary mesenchyme appear |
| 12:00 |
13 |
Late blastula 2 (mesenchymal blastula) |
| 15:00 |
14 |
Early gastrula 1. The beginning of invagination of the vegetative wall of the embryo |
| 17:00 |
15 |
Early gastrula 2. The appearance of the primordial bowel rudiment (archenteron) |
| 18:00 |
16 |
Middle gastrula 1. Archenteron reaches approximately the center of the blastocele. |
| 19:20 |
17 |
Average gastrula 2. Archenteron reaches its final size. |
| 20:30 |
18 |
Late gastrula 1. The emergence of the secondary mesenchyme and the beginning of the formation of ectodermal belts of cilia |
| 23:25 |
19 |
Late gastrula 2. The onset of skeletal formation |
| 25:00 |
20 |
Prism 1. Larvae take on a characteristic angular shape |
| 27:30 |
21 |
Prism 2. The digestive tract consists of poorly delimited anlages of the esophagus, stomach, and intestines |
| 29:50 |
22 |
Early pluteus 1. The mouth opening appeared. Chromatophores have appeared |
| 34:30 |
23 |
Early pluteus 2. The sections of the digestive tract are clearly delineated. |
| 39:35 |
24 |
Early pluteus 3. The first pair of arms increased noticeably, a bookmark of the second pair appeared - oral |
| 54:00 |
25 |
Middle pluteus 1. Both pairs of arms are extended |
| 84:00 |
26 |
Middle pluteus 2. Transition to active feeding |
*The stages of development of the hedgehogs that were used in work [
10] are marked in bold.
Table 1: Developmental stages of sea urchin [9].
Figure 1: Several of the 26 stages of sea urchin development.
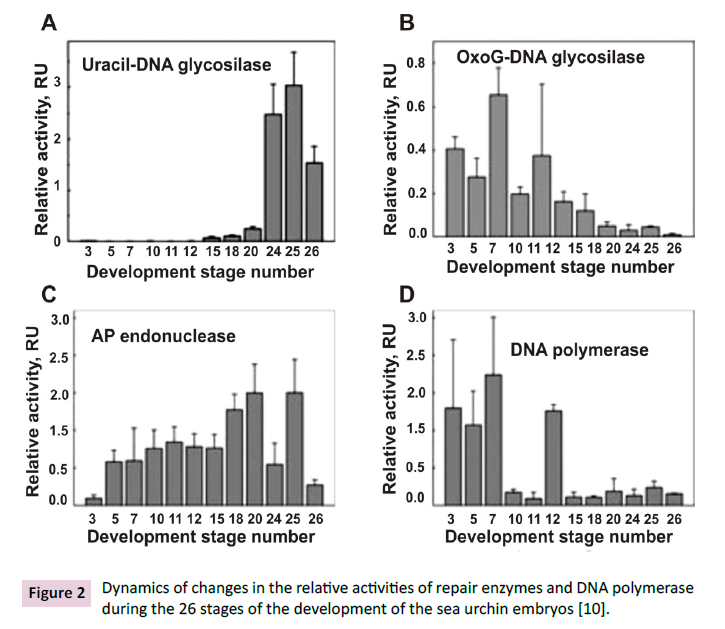
The relative activity of several repair enzymes was estimated by
oligonucleotide-based analysis, which is easy to interpret and oft used in DNA repair investigations in cell extracts of different organisms
[10]. Relative activity of uracil–DNA glycosylase, 8-oxoguanine–
DNA glycosylase, apurinic/apyrimidinic endonuclease,
and DNA polymerase during sea urchin embryo development was
analyzed. These sea urchin enzymes have not yet been described
in the literature.
The uracil–DNA glycosylase harshly upsurge after the prism stage
(Figure 2A), when the first signs of differentiation of the digestive
tract appear, while the specific activity of 8-oxoguanine–DNA
glycosylase (Figure 2B) constantly runs low over the course of
the development [10]. The AP endonuclease activity was gradually
increasing but fallen at the last stage mid-pluteus 2 (during
the transition of larvae to active feeding) (Figure 2C). At the first
stages of divisions (prior to completion of synchronous cleavage
of the cells), the DNA polymerase activity was high, and then it
decreases repeatedly, demonstrating a transient peak at stages
of late blastula after hatching (Figure 2D). The development of
sea urchin embryos seems to be depending on various factors of
DNA-damage in early stages within the protective envelope and
later as a free-floating larva, with hatching requiring adaptation to the shift in genotoxic stress conditions [10]. No correlation
was revealed between the enzymatic activity dynamics and the
known gene expression data from developing related species, Strongylocentrotus purpuratus [10]. The results suppose that the
base excision repair enzymes may be regulated in the embryos of
the urchins at the level of covalent modification or protein stability
[10]. It is possible that, in some sense, such changes in the
emergence or disappearance of repair enzymes can occur after
the fertilization of human cells. These data appear to be useful
for further investigation of DNA repair processes not only in the
case of sea urchins and other sea organisms but also in various
animals that may be very similar.
Figure 2: Dynamics of changes in the relative activities of repair enzymes and DNA polymerase
during the 26 stages of the development of the sea urchin embryos [10].
There is also some other evidence of DNA repair in sea urchins.
Treatment of sea urchin embryos with genotoxic agents (methyl
methanesulfonate or bleomycin) led to DNA damages and to the
cell cycle delay [11]. At low toxic agent doses, embryos were capable
to repair the DNA damage, while at high concentrations,
they show morphological and biochemical changes as well as
apoptosis. Embryos extracts obtained support in vitro repair of
oligonucleotides containing damages. The data demonstrate that
urchin embryos comprise enzymes and proteins important for
DNA repair [11].
The other approach used for the search of repair enzymes of sea
urchins is an investigation of effects of UV-irradiation, hydrogen
peroxide, etc., on larvae of the urchin Strongylocentrotus purpuratus.
Ultraviolet radiation effects on the urchin embryos and
led to the significant formation of DNA pyrimidine dimers [12].
Embryos of sea urchin after ultraviolet irradiation showed significantly
higher concentrations of proteins with antioxidant activities
and p53 and p21 transcription activators. The observed cellular
processes lead to apoptosis, and a significant increment in
DNA strands breaks in the nuclei of developing embryos treated
by ultraviolet light. Cell death and reduced survival of sea urchin
embryos are summoned by both indirect and direct effects of ultraviolet
radiation.
It was shown that ultraviolet radiation acts for proteins via a
few cellular pathways, which indicates that the mechanisms engaged
in UV-induced developmental delay in the urchin embryos
are integrated into multiple pathways for cellular stress, protein
turnover as well as translation, signal transduction, cytoskeletal
dynamics, and general metabolism [13]. The multiple responses of the urchin larvae to DNA damage may represent the specific
ways for protecting the population of these animals from acute
genotoxic stress.
The capability of the genome protection from harmful DNA damage
is very important to maintaining the stability of the genome
and defense against diseases. Many echinoderms, including sea
urchins, are marked by the absence of tumor diseases. In [14],
DNA damage was induced in coelocytes and larvae of adult sea
urchins under the influence of various genotoxicants, and the
ability to DNA repair was estimated during a 24-hour recovery
period. Larvae were more susceptible than coelocytes, with a
higher degree of initial DNA damage and increased mortality
levels for 24 hours after their treatment with different genotoxicants.
Surviving larvae were capable to repair damage within 24
hours effectively. Temporal expression profiles of 17 genes of
DNA repair proteins showed a large induction gene in whole cells
compared to larvae, with especially high expression of several of
them. Thus, DNA repair ability is very important for understanding
the effects of various genotoxicants on organisms and ways of
DNA recovery [14].
According to published data, there is evidence of changes in the
relative amount and activities of some other enzymes during embryonic
development. The turnover and localization of the DNAdependent
(cytosine-5)-methyltransferase were analyzed during
the development of the sea urchin embryo Paracentrotus lividus [15]. It was shown that the enzyme is, to varying degrees, needed
at various stages of embryonic development. The switching point occurs at the blastula stage, where the transferase presents in
all embryonic cells and removes a peptide of about 45 kDa from
the amino-terminal region of the 190 kDa protein. The 145 kDa
transferase form demonstrates its modified enzymatic properties,
and the enzyme is rapidly destroyed during several hours
before gastrulation. At later stages, the transferase is synthesized
again, but only in definite types of cells, including neurons. These
data demonstrate that enzyme concentration is differently critical
for different differentiated cells under a developing the urchin
embryo [15].
It was shown that the sea urchin Strongylocentrotus intermedius contains O-hydrosilhydrolases: very active 1,3-β-D-glycanase and
α-D-mannosidase and two enzymes with lower activities - β-Dglucosidase
and β-D-galactosidase [16]. The dynamics of change
in the activities of the enzymes at different stages of embryo
development were investigated. At all stages of embryo development,
a high level of α-D-mannosidase and 1,3-β-D-glycanase
was found. Activity β-D-glucosidase was low. 1,3-β-D-glycanase
was found in unfertilized eggs, and its activity decreased only
after the formation of 2, 4, and 8 blastomeres and at later
stages [16].
Proteolytic hydrolysis of sperm histones of male sea urchin occurs
due to the activation of maternal cysteine protease during
fertilization [17]. This protease is necessary for the remodeling
of male chromatin and for the development of the cell cycle in
lately formed embryos. This protease is presented in the nucleus
of unfertilized eggs and is promptly absorbed in the male pro-nucleus after insemination. This enzyme persists bound to chromatin
at the S phase of the first cell cycle; then, it moves to the
mitotic spindle in the M phase and, after cytokinesis, moves to
the nuclei of the daughter cells. A high identity of the protease
sequence with cathepsin proteolytic enzymes of different organisms
was found. Phylogenetic analysis clearly shows that this histone
sperm enzyme is a subtype of cathepsin L [17].
Thus, there are still relatively little data in the literature on various
repair and other enzymes that are involved in the development
of sea urchins. At the same time, this particular model is
extremely promising for studying the patterns of development
from embryo to the body. However, in the literature, there is data
on some other proteins and enzymes of sea urchins, which can be
used for understanding possible differences in the structure and
biological functions of their in marine organisms and in humans,
as well as for medicine and other purposes.
Sea Urchin Enzymes
The literature describes the features of the functioning of DNases
in humans and animals (e.g., [18-22]). These DNases are Mg2+-
dependent. However, DNases of sea urchins differ markedly from
those in mammals, which is of particular interest. Ca2+, Mg2+- and
Ca2+, Mn2+-dependent DNases were revealed in sea urchins. The
complexation between Ca2+, Mg2+-dependent DNase from sea urchin
embryos with double-stranded DNAs was shown using immunological
electron microscopy [18]. After the hydrolysis reaction,
in the DNA-DNase complex, the enzyme will stay bound with
the terminal fragment of DNA. It was shown that acid DNases
from marine organisms interact specifically with the local conformation
of B-DNA [19].
Ca2+, Mn2+ and Ca2+/Mg2+ dependent acid DNases were obtained
from spermatozoa of the sea urchin Strongylocentrotus intermedius
[6]. Ca, Mg-DNase is shown to be a nuclear protein with molecular
mass (MMs) ~63 kDa with pH-optimum at pH 7.5. The enzyme
activity declines in the following order: Ca2++Mg2+ > Mn2+ ≈
Ca2++Mn2+ > Mg2++EGTA > Ca2+. It has maximal activity in seawater.
The Ca,Mn-DNase (25 kDa optimal pH = 8.5) is answerable for the
internucleosomal splitting of spermatozoa chromosomal DNA.
The enzyme activity decreases in the following order: Ca2++Mn2+ > Ca2++Mg2+ > Mn2+ > Mg2++ EGTA. The enzyme is inactive in seawater.
Spermatozoa acid A-DNase with MM of 37 kDa (optimum
pH=5.5) is not a nuclear protein. A-DNase cannot be activated
by bivalent metal ions. Mechanisms of the hydrolysis of doublestranded
DNA were established for these three DNases.
Participation of Ca, Mg-DNase in spermatozoa apoptosis has
been shown using its strong inhibitor - Zn2+ ions [20]. It was demonstrated
that sphingolipid rhizochalin (Rhz) induced apoptotic
changes of nuclear chromatin, internucleosomal DNA cleavage,
and activation of several caspases in spermatozoa. In the presence
of Zn2+ ions, there was blocking of Rhz-induced DNA fragmentation
and internucleosomal cleavage of HeLa S and Vero E6
cell nuclei chromatin by highly purified Ca, Mg-DNase.
A new alkaline phosphatase (salt resistant, pH optimum = 8.0-
8.5, from eggs of the sea urchin Strongylocentrotus intermedius (StAP) was shown to have a unique property to split substrates in seawater with high activity [21]. Copper, zinc, cadmium, and
lead in different concentrations (15-150 μg/l) added to seawater,
or standard buffer completely suppress the phosphatase activity.
StAP is very sensitive to the presence in seawater of not only metals
but also different detergents, pesticides, and various oil products.
Seawater samples taken from aquatic areas of the Troitsy
Bay of the Peter the Great Bay of Japan Sea demonstrate inhibition
of the phosphatase activity; the same was demonstrated
for some samples of freshwaters. In contrast to phosphatases of
mammals, sea urchin enzyme suppression assay is highly sensitive
and technically easy-to-use, allowing testing a great number
of samples contaminations [21].
Later, it was shown that these alkaline phosphatase and Ca, Mg-
DNase inhibition tests are very productive for analysis of the total
pollution of natural marine ecosystems [22]. The sea water samples
containing various pollutions were getting together in different
places. The sensitivity of the alkaline phosphatase analysis of
water pollutes was comparable with the known standard sea urchin
sperm cell toxicity test. It was shown that a complex of these
two methods could be useful to evaluate marine water areas and
to estimate the biological conditions of invertebrates adapting to
various anthropogenic and environmental effects.
Several other sea urchins enzymes are described in the literature.
Gonad’s RNase of immature stage sea urchins showed a constant
level of activity [23]. RNase activity of mature males and females
of the sea urchin species Anthocidaris crassispina and Hemicentrotus
pulcherrimus (optimal pH = 5.0) demonstrated that its average
specific activity at the immature stage of the female H. pulcherrimus rapidly increased from 7.4 to 62.8 units/mg, while in
males H. pulcherrimus, it decreased from 7.4 to 1.9 units/mg. The
same phenomenon was observed in sea urchin A. crassispina. It
was determined that this sea urchin enzyme is an RNase T2
type [23].
Two different DNA ligases are described at the early sea urchin
embryogenesis [24]. Light form (50kDa) is revealed in unfertilized
eggs (oocyte form), and a heavier ligase (110kDa) is
found at the two-cell stage (embryonic form). α-Amylase is an
enzyme catalyzing the hydrolysis of starch and other polysaccharides
containing α-1,4-glycosidic bonds. Amylase is present in
some different organisms, where it carries out the processes of
hydrolysis of oligo- and polysaccharides. α-Amylase was isolated
from the digestive tract of sea urchins, Strongylocentrotus nudas
[25]. Chloride ions activate the enzyme and shift the optimal pH
from 6.0 (-NaCl) to 7.3 (50mM NaCl). The enzymatic properties of
sea urchin alpha-amylase were almost the same as in mammalian
α-amylases.
Cellulases are several enzymes produced chiefly by fungi, bacteria,
and protozoans, that catalyze the decomposition of cellulose
and of some related polysaccharides. Cellulases of the glycosidehydrolase-
9 family are known to be widespread in metazoan
[26]. Cellulase (54 kDa) was isolated from the Japanese purple
sea urchin Strongylocentrotus nudus, and its catalytic properties
and the primary structure of the protein were determined. The
enzyme was highly active in the hydrolysis of carboxymethyl cellulose
[26].
Sea urchin eggs activation at fertilization in deuterostomes needs
an increase in intracellular Ca2+, which is appeared from the egg's
endoplasmic reticulum [27]. In sea urchins, Src kinase (SpSFK1) is
necessary for a PLCgamma-associated signaling event that triggers
Ca2+ release. The function of the Src kinase in the initiation is
the release of Ca2+ during fertilization. The sequence analysis of
the Strongylocentrotus purpuratus genome results in the identification
of additional SFK kinases of Ca2+ transmission and activation
of sea urchin eggs. The cloning and analysis of four additional
SFKs and testing of their functions at the initial release of Ca2+ during fertilization were performed. While two new SFKs (SpFrk
and SpSFK3) are required for the release of Ca2+, SpSFK5 seems
unnecessary for the early events of transition from an egg to an
embryo. SpSFK7 may be involved in preventing the premature release
of Ca2+. The complexation analysis shows that only SpSFK1 is
able to bind directly with PLCgamma. One SpSFK and PLCgamma
are located according to immunolocalization studies in the cortex
of the egg and at the site of interaction between the sperm and
the eggs. Together, these data show that more than one SFK enzymes
are involved in the release of Ca2+ during fertilization [27].
The Strongylocentrotus purpuratus sea urchin sperm flagella creatine
kinase (CK) is both the main component of the sperm tail
membrane and cytosolic enzyme [28]. Three pools of a kinase
called CK I, CK II, and CK III were discovered. The relative activity
of these three types of kinases can be expressed approximately
as 1:10:1. However, they functionally differ in their ability to bind
to lipids. The data are consistent with the fact that the CK II association
with a membrane is a two-step process, including the
pH-dependent intramolecular process. The CK membrane association,
together with the microtubule association, may represent
the mechanism necessary for the specific accumulation of the
kinase in the flagellum [28].
In the sperm of the sea urchin, Strongylocentrotus purpuratus,
a functional shuttle of phosphocreatine, which is necessary for
the existence of mitochondrial and cytosolic isoforms in different
places, required for sperm motility [29]. Thus, the myristoylated
and non-myristoylated forms of creatine kinase exist side by side
in the flagellum of the sea urchin, and myristoylation is essential
for its efficient association with liposomes.
Multiprotein complex analysis
In living organisms, many protein complexes with very different
functions have been discovered [30]. Macromolecular complexes
are necessary for the retention of biological processes, but their
abundance among animals and other organisms is not yet fully
understood. In addition to the already discovered very stable
complexes of the ribosome and other type, biological fluids of
various organisms can contain other very stable complexes with
many different functions. Moreover, such complexes, which not
yet known in the literature, can be similar in structure and function
in mammals, marine and other organisms, or differ greatly.
Comparison of stable protein complexes from different organisms
can help to identify general and specific patterns of their existence
and biological function in organisms of different levels of
development and organization. Moreover, it is very possible that
some of the complexes can be detected at the beginning only using model animals like sea urchins and only then revealed in
humans.
By the combination of biochemical fractionations with quantitative
mass spectrometry, the analysis of soluble multiprotein complexes
in various multicellular animals was carried out [31]. Using
a combination of several approaches, a draft conservation map
was created that includes over one million predicted interactions
leading to the formation of various complexes with a high degree
of confidence. Clustering has revealed a wide spectrum of conservation,
ranging from ancient eukaryotic communities to rarer
innovations of multicellular animals. The completeness, centrality,
and modularity of these reconstructed interactions, according
to the author's point of view, demonstrate their fundamental
mechanistic importance and adaptive value for animal and other
organisms cell systems. However, the structure and biological
functions of real-life complexes can be established only after
their isolation and detailed analysis.
In comparison with individual proteins and enzymes, their complexes
usually possess polyfunctional properties and different
biological functions. The formation of stable complexes might be
an important system mechanism for the expansion of diversity
and the biological functions of different proteins and other substances
in different organisms, including humans. For example,
the majority of cellular processes are dependent on enzymes that
usually interact, forming larger temporary or stable protein complexes-
associates to increase the efficiency, specificity, and speed
of metabolic pathways [30].
At present, some new stable complexes of sea urchins and humans
have been obtained and characterized. We have analyzed
the likelihood of the presence of very stable protein complexes
in the placenta [32,33], milk [34,35] of women, and in the eggs
of sea urchins [36]. Previously undescribed highly stable protein
complexes were first found in the female placenta [32,33].
Human proteins associated with placental membranes were recently
analyzed by SDS-PAGE and MALDI mass spectrometry [37].
Overall, 733 unique proteins, including 34 protein complexes,
were identified. In our works, we searched for new stable complexes
in sea urchin eggs (Strongylocentrotus intermedius) not
described in the literature and compare them with human complexes.
The human placenta is a specific organ protecting, feeding, and
regulating the growth of the embryo. It appears that the revealing
and characterization of different components of the placenta,
including various proteins and possible multiprotein complexes,
is very important for understanding the placenta functions.
A very stable multiprotein complex (SPC, ∼1000 kDa) from the
soluble fraction of homogenates of human placentas [32,33] was
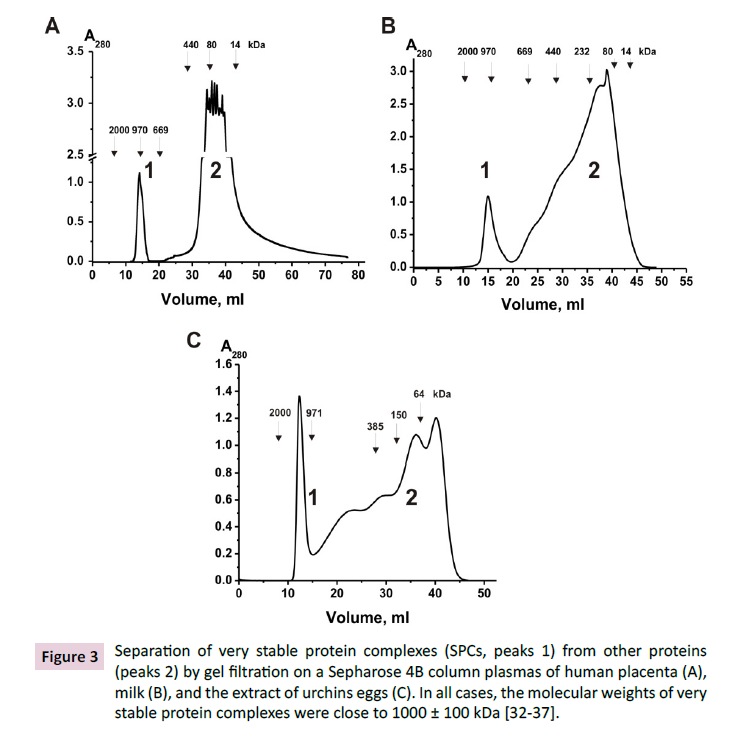
isolated. The SPCs were well separated by gel filtration on Sepharose-
4B from other proteins of the placental extracts (Figure
3A). The presence of stable multiprotein complexes was analyzed
in human milk [34,35] and eggs of sea urchins [36], which were
also separated by gel filtration (Figures 3B and 3C). In all cases,
separation of multiprotein complexes with molecular weights of
~1000±100 kDa occurred.
Figure 3: Separation of very stable protein complexes (SPCs, peaks 1) from other proteins
(peaks 2) by gel filtration on a Sepharose 4B column plasmas of human placenta (A),
milk (B), and the extract of urchins eggs (C). In all cases, the molecular weights of very
stable protein complexes were close to 1000 ± 100 kDa [32-37].
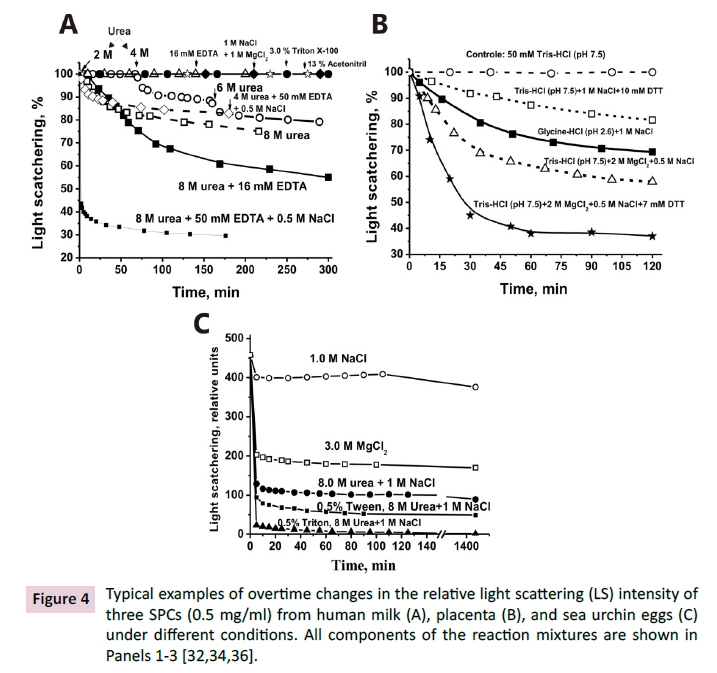
The analysis of complexes stability by light scattering (LS) demonstrated
that the SPCs are very stable in the presence of guanidinium
chloride, Triton X100, MgCl2, NaCl, acetonitrile, and other
compounds in high concentrations (Figure 4). The complexes dissociate
efficiently only in the presence of 8 M urea supplemented
with 0.5 M NaCl and 50 mM ethylenediaminetetraacetic acid
(EDTA) (Figures 4A and 4B) [33-35] or additionally with Tween or
Triton (Figure 4C) [36]. Such very stable complexes are unlikely
can be formed due to a random association of different proteins.
Figure 4: Typical examples of overtime changes in the relative light scattering (LS) intensity of
three SPCs (0.5 mg/ml) from human milk (A), placenta (B), and sea urchin eggs (C)
under different conditions. All components of the reaction mixtures are shown in
Panels 1-3 [32,34,36].
According to SDS-PAGE analysis and data of MALDI mass spectrometry,
the complex from the placenta contains many glycosylated
peptides and small proteins with 4-10 kDa MMs as well
as twelve proteins with higher MMs: alkaline phosphatase, hemoglobin,
chorionic somatomammotropin hormone, heat shock
protein beta-1, cytoplasmic actin, human serum albumin, peroxiredoxin-
1, 78 kDa glucose-regulated protein, disulfide isomerase
A3, serotransferrin, annexin A5, and immunoglobulin G (IgGs).
Each of these twelve proteins and enzymes has different and important
own biological functions in living organisms. It was shown
that the placenta SPC possesses two DNA-recognizing sites demonstrating
different affinities for a 12-mer oligonucleotide [32].
The placenta SPCs demonstrated nine different enzymatic activities:
catalase, peroxidase (H2O2-dependent), oxidoreductase (H2O2-independent), DNase, RNase, ATPase, phosphatase, protease,
and amylase [32,33]. The splitting of r(pC)23, r(pU)23, and
r(pA)23 by SPCs results the formation of 1-22-mer oligonucleotides,
while splitting of mirR137 results in its site-specific hydrolysis
at 3A-4U > 9U-10A > 8U-9U ≥ 15U-16A specific cleavage
sites. Some of these enzymatic activities of SPCs are absent in
the individual proteins included in the complex [32,33]. However,
new active centers of the complex can be formed at the junctions
of various proteins of the placenta SPC. The combination of
proteins with different functions in one complex can significantly
expand its biological functions due to the emergence of its multifunctionality.
For newborns, mother's milk is more than various nutrients
source; it provides a wide array of many different components
stimulating the growth of newborns and protecting children from
bacterial and viral infections. Therefore, the identification of female
milk components, including proteins and their complexes,
is very important for understanding milk functions. A very stable
multiprotein complex (SPC; ~1000 kDa) from 15 samples of
mother's milk was isolated as described above. The relative content
of the SPCs varied from 6 to 25% of the total different milk
proteins. Lactoferrin (LF) and α-lactalbumin were major proteins
of all fifteen SPCs, while human milk albumin and β-casein were presented as moderate or minor proteins; the relative content of
IgGs and secretory immunoglobulin A (sIgAs) varied greatly in different
SPC preparations. All SPCs efficiently split DNA and maltoheptaose
[34,35].
LF is known as the protein of the acute phase response and unspecific
defense against various types of viral and microbial infections
[34,35]. LF is found in human epithelial secretions, barrier
body fluids, and in the secondary granules of leukocytes. Therefore,
lactoferrin and its complexes with other proteins may be
formed not only in milk; they can be important for LF different
forms functioning not only in milk but also in other various biological
fluids and cells [34,35].
The association of enzymes and proteins can include different
metal ions, which may be essential for proteins complexations
and for their biological functions. However, there is no published
data concerning metal ions of any protein complexes. Content of
different microelements in the SPCs and milk was evaluated using
two-jet plasma atomic emission spectrometry [35]. The relative
content of various elements in mother’s milk decreased, on
average, in the following order: Ca > P > Mg > Al ≥ Zn ≥ Fe > Cu
> B (0.76-3500 μg/g of dry milk powder). The content of some
elements in milk was very low (Sr > Mn > Cr > Ba > Pb > Ag > Ni
> Cd; <0.03-0.5 μg/g). The relative amount of eight elements in
SPCs was by a factor of 1.2-9.6 higher than in females milk and
increased in the order: Ca ≈ Mg < P < Al < Fe < Pb < Ba < Cr < Cd < Zn. The content of eight metals in SPCs was 12.3-110-fold higher:
Cu (12.3) > B (19.7) > Ag (28.7) > Ni (38) ≥Sr (110) [35]. Thus, it
was shown that during the complexes formation, the increase in
the relative content of different metal ions in some complexes
is possible. Such an increase in the content of metal ions in the
complexes can be important for the processes of their association,
proteins, and enzymes conformations, their enzymatic activities,
and biological functions.
We have proposed that such very stable protein complexes can be
in different liquids and cells, including sea urchin eggs [36]. Identification
and characterization of embryonic peptides and proteins
and their different complexes seem to be very important for an
understanding of embryo proteins functions. Three preparations
consisting of a mixture of eggs of 10 different female sea urchins
were used [36]. Soluble fractions of the eggs homogenates were
obtained and subjected to gel filtration on the Sepharose 4B, as
described above (Figure 3C). A nearly symmetrical protein peak
with high molecular mass (~1100 ± 100 kDa) was separated from
other different peaks of proteins. According to the data of the LS
method, the sea urchin SPCs are stable in 20 mM Tris-HCl, pH 7.5,
containing 1 M NaCl, and to a lesser extent in the presence of 3
M MgCl2 usually destroying electrostatic contacts between different
proteins. Similar to very stable protein complexes from human
milk and placentas [32-34], the sea urchin eggs complex was
destroyed only by 8 M urea supplemented with 1.0-3.0 M NaCl, but better dissociated after the addition of Triton or Tween [37]
(Figure 4C). According to SDS-PAGE data, this complex contains
many major, moderate, and minor proteins with MMs from 10 to
95 kDa. Thus, all three complexes from different sources were of
approximately the same stability and comparable size.
MALDI-TOF mass spectrometry assay is a semi-quantitative approach
giving information mainly on only the MMs of analyzed
compounds. This method in the analysis of MMs of proteins mixtures
has some restrictions. If in the used conditions any of the
mixture’s protein is present in the increased concentration and
well crystallizes on MALDI-target, the signal of this protein can be
very high, when the signals of other proteins may be low or even
completely suppressed. Exactly this situation was observed during
the MALDI MS analysis of urchin SPCs.
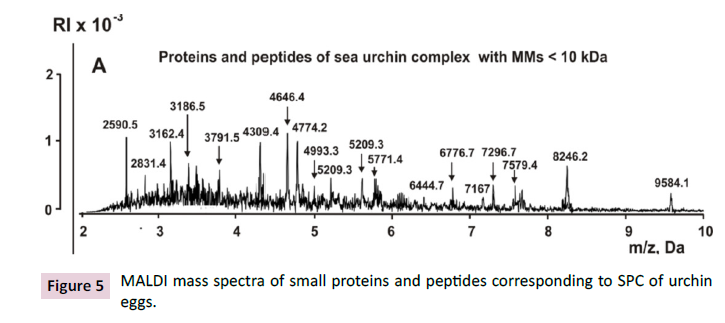
Proteins and peptides of lower MMs (<10 kDa) are usually soluble
in acetic acid, and during gel staining with Coomassie, they are
washed out of the gel. Therefore, three different sea urchin SPC
preparations were first boiled in buffer containing 1% SDS and
50 mM DTT, and then the samples were subjected to sequential
filtration using membranes skipping proteins with different MMs.
Finally, the fractions containing proteins ≤10 kDa were obtained
and analyzed using the MALDI mass spectrometry approach. The
fraction ≤10 kDa contains more than 20 sufficiently distinct peaks
of peptides and short proteins (Figure 5). Currently, evidence has
been obtained that SPCs of milk and the placenta, similar to sea
urchin complex, also contain many small proteins and peptides
(< 10 kDa).
Figure 5: MALDI mass spectra of small proteins and peptides corresponding to SPC of urchin
eggs.
The SPCs were first boiled in buffer containing 1% SDS and 50
mM DTT, and then the samples were subjected to sequential filtration
using membranes skipping proteins with MMs < 10 kDa.
Finally, these fraction components were analyzed using MALDI
mass spectrometry [36]. Furthermore, peaks correspond to the
fraction of 10-30 kDa. Fraction ≤100 kDa contains approximately
20 proteins with MMs 29.4-96.1 kDa [36].
Thus, SPCs consist of many proteins and peptides. The small gel
fragments after SDS-PAGE corresponding to major and medium
proteins were isolated. We tried to identify these proteins by
MALDI mass spectrometric analysis (MS and MS/MS) of their tryptic hydrolyzates. All proteins of SPCs from milk and the placenta
were successfully identified (see above).
Good MS and MS/MS spectra were obtained for several urchin
SPC proteins. It was impossible, however, to identify the majority
of the proteins due to the lack of data on such proteins in the sea
urchins and other invertebrate’s databases. Assuming possible
homology of proteins of the sea urchin eggs and other organisms,
we used other databases. One protein has homology with keratin
(type II cytoskeletal 1; Homo sapiens). The second one was
homologous to alkaline phosphatase (Pseudomonas fluorescens),
while the third protein demonstrated homology with human lactotransferrin.
A homolog of glucose-6-phosphatase of Strongylocentrotus
purpuratus corresponds to the fourth protein, while
the homolog of the Alveinella pompejana protein (cDNA clone
CAGA18424 5’, mRNA sequence) to the fifth protein. Other medium
and major proteins were impossible to identify even by their
potential homology with proteins of other various organisms.
The presence in the placenta [33] and sea urchin [36] native
SPCs of alkaline phosphatase was additionally confirmed by the
detection of these complexes’ phosphatase activity. Thus, it was
shown, for the first time that sea urchin's eggs similar to human
milk and placenta contain a very stable protein complex of
high MM ≈ ~1100 kDa consisting of many peptides and proteins
[34,35]. Thus, only five proteins of sea urchin's eggs SPCs were
identified. In addition, to large proteins SPCs contain many different
small proteins and peptides with MMs from 2 to 9.5 kDa. It
should be especially noted that very stable complexes have been
found in both humans and sea urchins. However, these complexes
contain different peptides and proteins. This suggests that they
can have very different functions in these organisms. Analysis of
their possible biological functions looks very important [36,37].
Conclusion
This review combines for the first time the available results of
the study of various enzymes, proteins, peptides, and their stable
complexes from sea urchin, the analysis of which was only possible
due to cooperation between two laboratories of two different
institutes.
Acknowledgments
This collaborative research of two laboratories of ICBFM and PIBOC was partially supported by grants from the Russian Foundation
for Basic Research to Dmitrenok P.S (18-04-00442 А.) and
to Soboleva S.E (20-04-00373).
References
- Fuji A (1960) Studies on the biology of the sea urchin superficial and histological changes in gametogenic process of two sea urchins Strongylocentrotus nudus and S Intermedius. Bull Fac Fish Hokkaido Univ11: 1–14.
- Lawrence JM (2001) Sea urchins: Biology and ecology (3rd edn) Academic Press of Elsevier.
- Lawrence JM (2013) Edible Sea Urchins: Biology and Ecology Elsevier Science, An imprint of Elsevier.
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, et al. (2006) DNA repair and mutagenesis. ASM Press Washington DC, USA.
- Friedberg EC, Meira LB (2006) Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage Version 7. DNA Repair5: 189–209.
- Menzorova NI, Rasskazov VA (1980) Effect of bivalent metal ions on enzymatic activity of Ca2+, Mg2+-dependent DNase from sea urchin Stronglyocentrotus intermedius embryos. Biokhimiia 45: 544–553.
- Shastina VV, Menzorova NI, Sibirtsev YT, Rasskazov VA (2003) Purification and characteristics of Ca2+, Mg2+ and Ca2+, Mn2+-dependent and acid DNases from spermatozoa of the sea urchin Strongylocentrotus intermedius Biochemistry (Mosc). 68: 582–592.
- Wessel GM, Vacquier VD (2004) Isolation of organelles and components from sea urchin eggs and embryos Methods Cell Biol74: 491–522.
- Buznikov GA, Podmarev VI (1975) Objects of Developmental Biology by Astaurov BL ed., Nauka, Moscow, pp. 188-216.
- Torgasheva NA, Menzorova NI, Sibirtsev YT, Rasskazov VA, Zharkov DO, et al. (2016) Base excision DNA repair in the embryonic development of the sea urchin Strongylocentrotus intermedius, Mol Biosyst12: 2247–2256.
- Le Bouffant R, Cormier P, Cueff A, Bellé R, Mulner-Lorillon O (2007) Sea urchin embryo as a model for analysis of the signaling pathways linking DNA damage checkpoint DNA repair and apoptosis. Cell Mol Life Sci 64: 1723-1734.
- Lesser MP, Kruse VA, Barry TM (2003) Exposure to ultraviolet radiation causes apoptosis in developing sea urchin embryos J Exp Biol 206: 4097-4103.
- Campanale JP, Tomanek L, Adams NL (2011) Exposure to ultraviolet radiation causes proteomic changes in embryos of the purple sea urchin Strongylocentrotus purpuratus. J Exp Mar Bio Ecol 397: 106–120.
- Reinardy HC, Bodnar AG (2015) Profiling DNA damage and repair capacity in sea urchin larvae and coelomocytes exposed to genotoxicants. Mutagenesis 30: 829–839.
- Di Giaimo R, Locascio A, Aniello F, Branno M, Del Gaudio R, et al. (2001) DNA (cytosine-5) methyltransferase turnover and cellular localization in developing Paracentrotus lividus sea urchin embryo. Gene 272: 199-208.
- Verigina NS, Kiseleva MI, Ermakova SP, Sova VV, Zvyagintseva TN (2009) O-glycosylhydrolases of embryos of the sea urchin Strongylocentrotus intermedius and effect of some natural substances on their biosynthesisZh Evol Biokhim Fiziol45: 53-58.
- Morin V, Sanchez-Rubio A, Aze A, Iribarren C, Fayet C, et al. (2012) The protease degrading sperm histones post-fertilization in sea urchin eggs is a nuclear cathepsin L that is further required for embryo development. PLoS One 7: e46850.
- Abramova ZI, Kosareva TI, Vinter VG (1995) The interaction of the Ca2+-and Mg2+-dependent DNases of sea urchin embryos with DNA.Tsitologiia 37: 893-899.
- Rasskazov VA, Kozhemiako VB (1987) Specificity of acid DNases from marine organisms to local conformation of B-DNA. Dokl Akad Nauk SSSR 294: 1497-1500.
- Sibirtsev JT, Shastina VV, Menzorova NI, Makarieva TN, Rasskazov VA (2011) Ca2+, Mg2+-dependent DNase involvement in apoptotic effects in spermatozoa of sea urchin Strongylocentrotus intermedius induced by two-headed sphingolipid rhizochalin. Mar Biotechnol 13: 536-543.
- Menzorova NI, Seitkalieva AV, Rasskazov VA (2014) Enzymatic methods for the determination of pollution in seawater using salt resistant alkaline phosphatase from eggs of the sea urchin Strongylocentrotus intermedius. Mar Pollut Bull 79: 188-195
- Seitkalieva AV, Menzorova NI, Rasskazov VA (2016) Application of different enzyme assays and biomarkers for pollution monitoring of the marine environment. Environ Monit Assess 188: 70.
- Sanda A, Kiyomoto M, Iwama M, Ohgi K, Irie M (2008) Marked changes in the ribonuclease activity of mature and immature gonads of sea urchins Hemicentrotus pulcherrimus and Anthocidaris crassispina. Biol Pharm Bull 31: 1659-1662
- Prigent C, Maniey D, Lefresne J, Epel D, Signoret J, et al. (1987) Changes in the catalytic properties of DNA ligases during early sea urchin development. Dev Biol 124: 281-286.
- Nacatany H, Kobayashi I (1996) Enzymatic properties of α-amylase from sea urchin from Strongulocentrotus nudas. Comp Biochem Physiol 113B: 383-386.
- Nishida Y, Suzuki K, Kumagai Y, Tanaka H, Inoue A, et al. (2007) Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochimie 89: 1002-1011.
- Townley IK, Schuyler E, Parker-Gür M, Foltz KR (2009) Expression of multiple Src family kinases in sea urchin eggs and their function in Ca2+ release at fertilization. Dev Boil327: 465-477.
- Quest AF, Harvey DJ, McIlhinney RA (1997) Myristoylated and nonmyristoylated pools of sea urchin sperm flagellar creatine kinase exist side-by-side: Myristoylation is necessary for efficient lipid association. Biochemistry 36: 6993-7002.
- Quest AF, Shapiro BM (1991) Membrane association of flagellar creatine kinase in the sperm phosphocreatine shuttle. J Biol Chem 266: 19803-19811.
- Eubel H, Braun HP, Millar AH (2005) Blue-native PAGE in plants: A tool in analysis of protein-protein interactions. Plant Methods1: 11.
- Wan C, Borgeson B, Phanse S, Tu F, Drew K, et al. (2015) Panorama of ancient metazoan macromolecular complexes. Nature 525: 339-344.
- Burkova EE, Dmitrenok PS, Sedykh SE, Buneva VN, Soboleva SE, et al. (2014) Extremely stable soluble high molecular mass multi-protein complex with DNase activity in human placental tissue. PLoS One9: e111234.
- Burkova EE, Dmitrenok PS, Bulgakov DV, Ermakov EA, Buneva VN, et al. (2018) Identification of major proteins of a very stable high molecular mass multi-protein complex of human placental tissue possessing nine different catalytic activities. Biochem Anal Biochem 7: 351.
- Soboleva SE, Dmitrenok PS, Verkhovod TD, Buneva VN, Sedykh SE, et al. (2015) Very stable high molecular mass multiprotein complex with DNase and amylase activities in human milk. J Mol Recognit28: 20–34.
- Soboleva SE, Zaksas NP, Nevinsky GA (2019) Comparison of trace elements in high-molecular-mass multiprotein complex and in female milk from which it was obtained. ScientificWorldJournal 2578975.
- Soboleva SE, Burkova EE, Dmitrenok PS, Bulgakov DV, Menzorova NI, et al. (2018) Extremely stable high molecular mass soluble multiprotein complex from eggs of sea urchin Strongylocentrotus intermedius with phosphatase activity. J Mol Recognit 31: e2753.
- Wang F, Wang L, Liang G (2013) Identification and analysis of multi-protein complexes in placenta. PLoS One138: e62988.