Research Article - (2015) Volume 1, Issue 1
Goutam Brahmachari*
Laboratory of Natural Products & Organic Synthesis, Department of Chemistry, Visva-Bharati (a Central University), Santiniketan-731235, West Bengal, India
Corresponding Author:
Goutam Brahmachari
Laboratory of Natural Products & Organic Synthesis
Department of Chemistry, Visva-Bharati (a Central University)
Santiniketan-731235, West Bengal, India
Tel: +91(3463)262751
E-mail: brahmg2001@yahoo.co.in
Among various green chemistry aspects, selection of catalysts for smooth running of a particular reaction with optimum yield is an important part of the chemical process. In addition to develop novel catalytic systems (homogeneous and heterogeneous catalysts) including nanomaterials and polymer grafting, still there is a tremendous scope in screening commercially available low-cost and less-toxic agents that can carry out an organic transformation of choice in an efficient manner under eco-friendly conditions. Synthetic chemists are engaged to explore this area of interest as one of the current trends in green chemistry, and as a result many such simple chemical entities have now appeared as promising catalysts in effecting a considerable number of organic reactions. Sodium formate is a low-cost commercially available substance which is reported as a less-toxic substance. We have recently demonstrated for the first time that sodium formate can be used as an efficient catalyst for smooth running of some organic transformations under eco-friendly conditions. This article sum-ups our experimental results on the catalytic efficacy of sodium formate as an eco-friendly catalyst in case of certain organic transformations of interest.
Keywords
Sodium formate, Eco-friendly catalyst, Organic transformations, N-formamides, 4H-pyrans, 4H-pyran annulated, Benzopyrano[2,3-d] pyrimidines, 4-thio-substituted 4H-chromenes
Introduction
The world of organic compounds is an outcome of the continuing endeavor of organic chemists during hundreds of years, and the synthetic protocols developed by them have been at the core of the chemical industry in the production of a plethora of organic compounds finding immense applications as fine chemicals, medicinal and pharmaceutical agents, agrochemicals, and many others [1]. However, with the advent of the twenty-first century, the public is equally aware of the hazardous substances used and generated by the chemical processes, and eventually the ‘concept of green and sustainable chemistry’ has been evolved. The main essence of this concept is to develop a sustainable chemical enterprise that will find creative ways to minimize human exposure to, and the environmental impact of, harmful chemicals while enhancing scientific progress. Current trends of green chemistry practice encompass a number of agenda such as the avoidance of extensive use of toxic and hazardous reagents and solvents, harsh reaction conditions, and expensive and sophisticated catalysts [2-4]. The last decade has seen a tremendous effort toward savings in energy consumption, use of eco-friendly catalysts and solvents, proficiency in atom economy, and minimization of wastes from reactions in designing novel green synthetic protocols for organic compounds of interests [1-4].
Catalysts play a pivotal role in most of the organic reactions and it is, thus, a field of topical interest. In addition to develop novel catalytic systems (homogeneous and heterogeneous catalysts) including nano materials and polymer grafting, still there is also a tremendous scope in screening commercially available low-cost and less-toxic agents that can carry out an organic transformation of choice in an efficient manner under eco-friendly conditions [5-8]. Synthetic chemists are engaged to explore this area of interest as one of the current trends in green chemistry, and as a result many such simple chemical entities have now appeared as promising catalysts in effecting a considerable number of organic reactions. As part of our ongoing endeavor in the field of green chemistry practice, we have recently demonstrated commercially available low-cost and less-toxic sodium formate, for the first time, as an efficient catalyst in carrying out couples of reactions. The clinical and toxicity evaluation of sodium formate in humans are indicative of its less-toxicity [9-11]. The present article highlights on our experimental results with sodium formate that has come out as an efficient and eco-friendly catalyst in carrying out certain organic transformations of interest.
Catalytic Application of Sodium Formate: A Test Case
As mentioned above, sodium formate is commercially easily available, cheap and less-toxic chemical substance. As part of our continuing endeavor to develop green synthetic protocols for important organic transformations, we have been motivated to screen this eco-friendly chemical entity to explore its possible application as a basic catalyst. To our delight, we have been successful in demonstrating efficient catalytic performance of this low-cost and low-toxic chemical substance, for the first time, in three organic transformations of potential interest so far. Herein, we wish to offer to a brief overview of our experimental outcomes.
N-Formylation of Primary and Secondary Amines
N-Formylation of amines is an important reaction in organic chemistry. Formamides are widely used in the synthesis of pharmaceutically valuable compounds such as fluroquinolones, substituted aryl imidazoles, 1,2-dihydroquinolines, nitrogen bridged heterocycles, functionalized peptide derivatives, oxazolidinone and cancer chemotherapeutic agents [12-18]. They also constitute important precursors in the synthesis of fungicides and herbicides [13]. In addition, formamides are very useful reagents in Vilsmeier formylation reactions as well as in the synthesis of formamidines and isocyanides [19-21]. Such immense applications of formamides in diverse fields, synthetic chemists developed a number of formylation methods in the recent past, describing on alternative formylating agents under different reaction conditions, although acetic formic anhydride still continues to be the most widely used formylating reagent in spite it is being sensitive to atmospheric moisture and cannot be stored due to decomposition to acetic acid and carbon monoxide [22-29]. On critical survey of these earlier methods, it has been revealed that most of them suffer from genuine drawbacks such as use of expensive and toxic formylating agents and catalysts, use of organic solvents, high temperature, long reaction time and the removal of by-products. Under this purview, we have been motivated to design a novel reaction protocol as an alternative route of N-formylation of amines to get rid of these shortcomings, and developed a convenient and highly efficient protocol for N-formylation of a variety of structurally diverse primary and secondary amines (1) at room temperature in excellent yields, involving the application of a catalytic amount of sodium formate (a readily available, inexpensive and nontoxic substance) in formic acid (2) under solvent-free conditions [30]. The process is remarkably simple, highly efficient and environmentally benign (Figure 1).
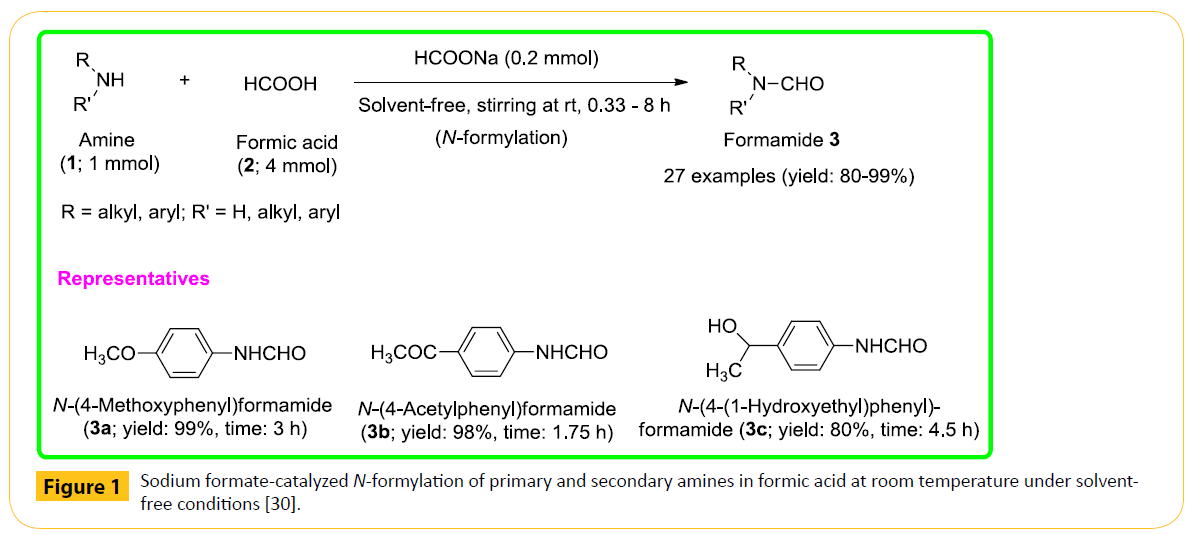
Figure 1: Sodium formate-catalyzed N-formylation of primary and secondary amines in formic acid at room temperature under solventfree conditions [30].
A good of number of structurally diverse primary and secondary amines (aliphatic, aromatic and heterocyclic) underwent the N-formylation reaction smoothly under the optimized conditions producing the corresponding N-formyl derivatives 3 with good to excellent yields. Anilines bearing both electron-donating and electron-withdrawing functionalities were also found to undergo the conversion in a facile manner. Previously, the N-formylation of anilines having electron-withdrawing groups was found to be difficult. The present method is completely chemoselective and alcoholic or phenolic-OH group within the substrates was found to be well-tolerated without yielding any O-formylated product. The workup of the reaction mixtures is simple and highly convenient, and the products are in many cases obtained in high purity. Furthermore, sodium formate used as a catalyst in the formylation reaction can also be recovered and reused.
We assume that the sodium cation resulting from the ionization of sodium formate in the reaction mixture attaches itself with the carbonyl oxygen of the carboxylic functionality of formic acid, thereby, enhancing the electron-deficiency of the attacking site (carbonyl carbon); hence, the nucleophilic attack by an amine is facilitated so effectively that the formylation reaction goes on smoothly at room temperature. Besides, it may also be suggested that the formate ion eventually acts as a base and removes the proton from the resulting dialkylammonium moiety. The key advantages of this environmentally benign and safe protocol include a simple reaction setup at room temperature, not requiring specialized equipment, high product yields, the possibility for reusing the catalyst, chemoselectivity, and solventfree conditions.
Synthesis of 4H-pyrans and 4H-pyran Annulated Heterocycles
4H-Pyrans and 4H-pyran annulated heterocyclic scaffolds represent a “privileged” structural motif well-distributed in naturally occurring compounds with a broad spectrum of significant biological activities that include anticancer, cytotoxic, anti-HIV, anti-inflammatory, antimalarial, antimicrobial, antihyperglycemic and antidyslipidemic, anti-neurodegenerative disorders like Alzheimer’s, Parkinson disease and Huntington’s disease, and many more [31-35]. Moreover, functionalized 4H-pyran derivatives have played increasing roles in synthetic approaches to promising compounds in the field of medicinal agrochemical cosmetics and pigment industries [36-39]. It is worthy to mention that currently, a number of drug molecules bearing 4H-pyran moiety are in use in the treatment of various ailments, such as hypertension, asthma, ischemia, and urinary incontinence [40-43]. In addition, such 4H-pyran derivatives are also administrated to animals suffering from a disorder responsive to the positive modulation of AMPA receptor as effective remedy [44]. 2-Amino-3-cyano-4H-pyrans are found to exhibit significant photochemical activity as well [45]. Recently, a series of synthetic 2-amino-3-cyano-4H-pyrans have been evaluated to possess potent anticancer, antibacterial and antifungal, and anti-rheumatic properties. Besides, the 4H-pyran ring can be transformed to dihydropyridine (DHP) type systems having promising calcium antagonist properties [46-55]. Such handful of diverse applications of 4H-pyrans and pyran annulated heterocyclic scaffolds in medicinal chemistry have drawn considerable interest during the last several years among the synthetic chemists to develop useful synthetic routes to these high valued-added heterocycles.
As part of our interest in developing room temperature procedures for important organic transformations, we have been able to design a one-pot multicomponent reaction (MCR) strategy for synthesizing these scaffolds under green conditions. In 2014, we have reported a very simple, facile and convenient practical method for one-pot synthesis of diverse kind of pyran annulated heterocycles such as 4H-pyrano[3,2-c]coumarins (7a), 4H-pyrano[3,2-c]-α-pyrones (7b), 5,6,7,8-tetrahydro-5-oxo-4Hchromenes (7c) and benzo[h]-4H-chromenes (7d) in the presence of sodium formate as catalyst via three-component condensation reaction of aromatic aldehydes (4), malononitrile (5) and C-H activated acidic compounds (6) in aqueous ethanol mostly at ambient conditions (Figure 2) [56]. Mild reaction conditions, good to excellent yields, operational simplicity and absence of tedious separation procedures (no column chromatographic purification), clean reaction profiles, high atom-economy as well as the use of inexpensive and environmentally benign catalyst are the key advantages of the present method.
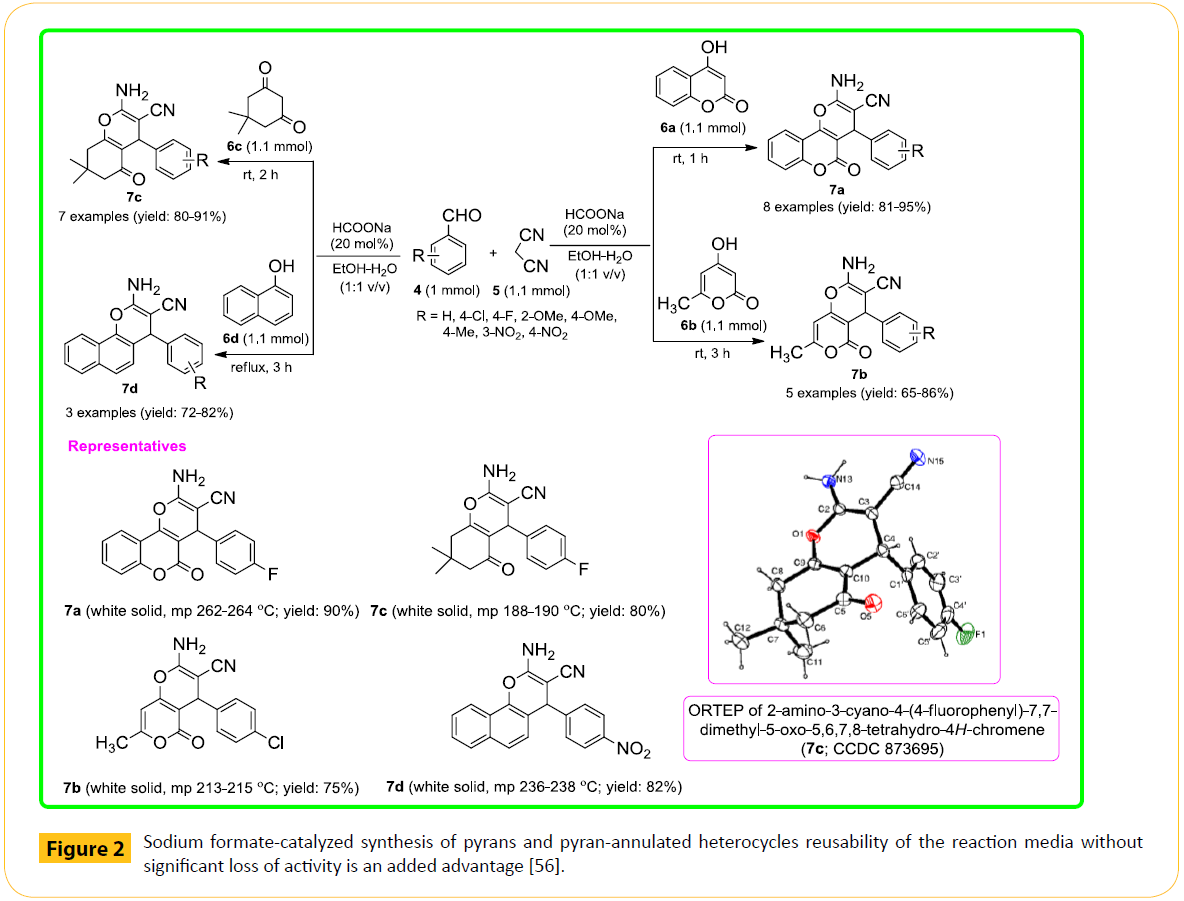
Figure 2: Sodium formate-catalyzed synthesis of pyrans and pyran-annulated heterocycles reusability of the reaction media without significant loss of activity is an added advantage [56].
Synthesis of Benzopyrano[2,3-d] Pyrimidines and 4-thio-substituted 4H-Chromenes
Moreover, Benzopyrano[2,3-d]pyrimidines exhibit a wide range of pharmaceutical potentials that include anti-inflammatory, analgesic, and anti-aggregating activities more importantly, such chemical entities have been found to possess potent in vivo antitumor as well as in vitro cytotoxic activity against various cancer cell lines causing considerable degree of perturbation in cell cycle kinetics [57,58]. Benzopyrano[2,3-d]pyrimidine core was first synthesized by O’Callaghan from the condensation reaction of 2-iminocoumarin-3-carboxamides with aldehydes following a complex multi-step protocol [59,60]. Later on, a few more reports were appeared describing the synthesis of benzopyranopyrimidine derivatives with a limited substitution pattern via multistep complex reaction procedures [61-64]. However, one-pot multicomponent synthetic protocols for such scaffolds have recently been reported based on the use of microwave irradiation or catalysts such as lithium perchlorate and the ionic liquid [Bmim]BF4 despite such sincere attempts to overcome the existing drawbacks, no one of these three procedures is sufficient enough to attain ‘green credentials’ for synthesizing this core [65].
Hence, we have been motivated to develop a clean and efficient protocol for the synthesis of benzopyrano[2,3-d]pyrimidines through a more convenient and eco-friendly manner, just using simple sodium formate as an efficient catalyst. Our recent report is based on the development of a straightforward and efficient protocol for one-pot synthesis of benzopyrano[2,3-d]pyrimidine scaffolds (10) in the presence of sodium formate as catalyst via pseudo-four component tandem reaction of salicylic aldehydes (8), malononilrile (5) and cyclic secondary amines (9) at room temperature. The reaction has also been extended to the synthesis of 2-amino-4-arylsulfanyl-4H-chromene-3-corbonitriles (12), another important group of heterocycles [66]. The overall reaction profile is shown in Figure 3.
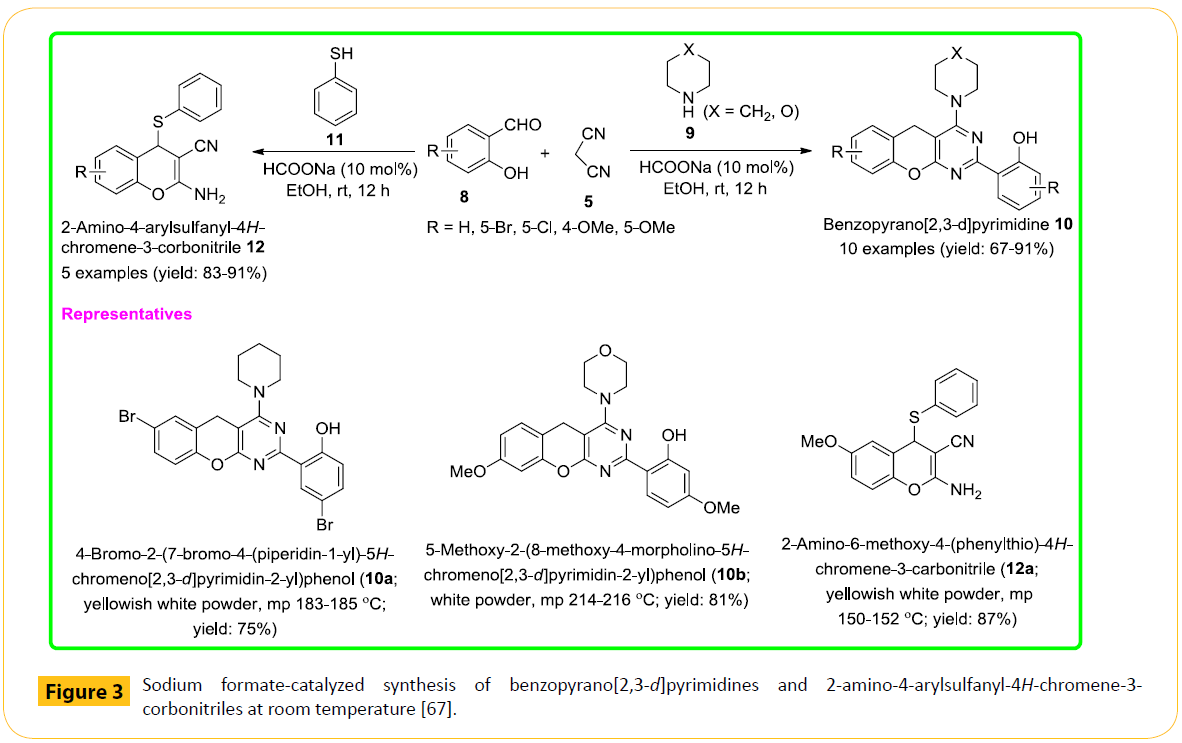
Figure 3: Sodium formate-catalyzed synthesis of benzopyrano[2,3-d]pyrimidines and 2-amino-4-arylsulfanyl-4H-chromene-3-corbonitriles at room temperature [67].
We have proposed a mechanism in Figure 4 for the present reaction leading to the formation of benzopyrano [2,3-d] pyrimidine (4). Initially, the Knoevenagel adduct (14) is formed via condensation of the salicylic aldehydes (8) with malononitrile (5) with the catalyst, sodium formate, acting as the base. The adduct 14, thus formed in situ, undergoes ring closure to give 2-imino-3- cyano-2H-chromene (15; isolated) in ethanol medium. In the very next step, possibly the cyano group of intermediate 15 is attacked by the nucleophilic cyclic secondary amine (9) affording 16, which reacts with another molecule of salicylic aldehyde (8) involving rapid proton transfer under the reaction conditions to furnish benzopyrano[2,3-d]pyrimidine (10). In support of our prediction, we isolated and characterized the intermediate 15 from our model reaction, which yielded the expected product on reaction with piperidine (9) and another molecule of salicylaldehyde (8). Again, formation of 4H-chromene (12) could be rationalized out of the possible nucleophilic attack at C-4 of 2-imino-3-cyano-2H-chromene intermediate (15), when a relatively less nucleophilic thiophenol (11) was used.
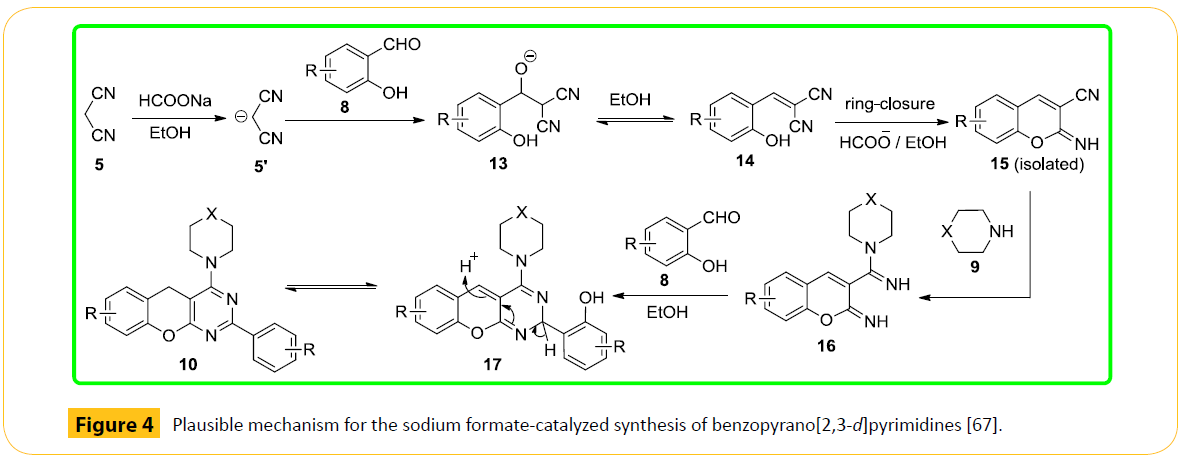
Figure 4: Plausible mechanism for the sodium formate-catalyzed synthesis of benzopyrano[2,3-d]pyrimidines [67].
It is worth noting that we reused the filtrate containing residual solvent, catalyst and substrates, obtained upon filtration of the reaction mixture after completion of the reaction up to 4th times in case of a representative entry. Mild reaction conditions, good yields, operational simplicity and absence of tedious separation procedures, clean reaction profiles, high atom-economy, inexpensive starting materials, and environmentally benign catalyst are the key advantages of the present protocol.
Conclusion
Thus we have demonstrated the efficient catalytic proficiency of easily available commercial sodium format as a basic catalyst, for the first time, in carrying out certain organic transformations involving both carbon-carbon and carbon-heteroatom bond formations under mild reaction conditions. A variety of biologically relevant organic scaffolds including heterocycles have been synthesized under sodium formate catalysis with excellent yields. The major strength of the newly developed synthetic protocols using this inexpensive and less-toxic catalyst lies in the fact that they satisfy a number of green chemistry requirements such as the use of less-toxic and low-cost catalyst, avoidance of toxic organic solvents, operational simplicity, avoidance of tedious column chromatographic purification, reuse of reaction media, excellent yields, high atom-economy, and mild reaction conditions. We have just initiated the catalytic exploration of this eco-friendly chemical substance, and we are sure the future will see more such developments! Hope that this commentary would boost the ongoing development in this direction.
Acknowledgment
The author is thankful to the DST, New Delhi (Grant No. EMR/2014/001220) for financial support.