Research Article - (2015) Volume 1, Issue 1
Kyzas GZ, Deliyanni EA , Matis KA*
Laboratory of General & Inorganic Chemical Technology, Division of Chemical Technology, Department of Chemistry, Aristotle University of Thessaloniki, Thessaloniki, Greece
Corresponding Author:
Matis KA
Department of Chemistry, Aristotle University of Thessaloniki
P.O. Box: 116, Postal code: 54124, Thessaloniki, Greece
Tel: +30-2310-997743
E-mail: kamatis@chem.auth.gr
Flotation initially originated from the field of mineral processing. The importance of this process to the economy of the whole industrial world is considered to be enormous. Since for many years various particulate solids besides minerals have been extracted by using this effective gravity separation method with many interesting applications in the treatment of wastewater, looking for sustainability. Certain significant activities in flotation research were reviewed in the present, with main focus in the contribution of physical chemistry to flotation; i.e. zeta-potential measurements, contact angle etc., including examining the role of bubble and particle size played with regard to the process. Alternative bubble generation methods were examined, such as electro flotation and dissolved-air flotation-the latter technique is mainly intended for water treatment applications.
Keywords
Hydrophobicity, Dissolved-air flotation, Electroflotation, Biosorption, Waste water
Introduction
Let us start this chapter by saying that flotation is really an old process: the historian Herodotus in ancient Greece is probably the first person that described a process similar to flotation, in the 5th century B.C., for the separation of gold particles from sand using fatty substances. And later, in the 15th century A.D., the Arabs used resins for the selective separation of azurite (a copper mineral) from gangue. These are considered to be the main historical roots of flotation, which is based on the idea of applying rising gas bubbles as the transport medium [1]. The attachment of bubbles to particles transfers the solids from the body of water to the surface.
Hydrophobicity refers to the property of a molecule, cluster, micro-organism or mineral surface that is repelled from a mass of water. Further, it was noted that the term hydrophobicity (a Greek word) has been differently understood in various fields [2]. Floatability and hydrophobicity, however, are inter-related, focusing often on the application of collectorless flotation. Attention may be paid to a series of relevant reviews on the area [3-6]. Below a brief survey is given on selected recent papers for different applications of flotation, from a really vast literatureshowing well (as we believe) that chemistry contributes in various ways in flotation research. Coal beneficiation is one of the most effective methods for removing minerals, such as gangues and pyrite, and pollutants such as sulfur, before the burning of coal.
In general, the beneficiation process of low rank coals (about the 50% of the world's total coal deposits) is more difficult to achieve than that of bituminous and/or anthracite coals. Lignite is the most important mineral commodities for Greece, according to Anastassakis [7]; its reserves approach to about 4000 Mt. It was pointed out that in the future effective reagents should be invented for effective flotation process (being a physico-chemical one) [8]. A better understanding of the surface chemistry of sulfide– water interfaces was tried, to improve froth flotation at industrial scale [9]. Zeta-potential measurements were used to assess the electrokinetic characteristics of chalcopyrite, molybdenite and pyrite in the presence of main components (such as humic acids, glucose and serum albumin) and a commercial collector. Fourier transform infrared spectroscopy was then used to gain a deeper understanding of the interaction of these compounds with the sulphide minerals.
The success of mineral flotation is contingent upon the interfacial interaction between the collector(s) and the mineral surface, was also argued in another research article [10]. In order to characterize and ascertain the mechanisms of flotation reagent interaction with rare earth oxide minerals, it was necessary to determine the physical and chemical properties of the constituent components applying Raman and fluorescence spectroscopy.
Metallurgical and mineral processing industries generate a huge amount of wastes in the form of fines, slimes, slag, sludge etc.; the primary aim has been directed towards the development of a zero waste technology by the utilization of such by-products or other industrial wastes. So, copper slag was subjected to indepth mineralogical characterization by integrated instrumental techniques and evaluated for the efficacy of physical beneficiation and mixed meso-acidophilic bioleaching tests towards recovery of copper [11].
The toxicity of heavy metals, which possibly may be accumulated through the food chain, is associated with metal ion speciation; controlling heavy metal discharges and their removal from aqueous solutions have become a challenge. Cu2+ removal from aqueous systems, by combining biosorption onto inactive dry baker’s yeast Saccharomyces cerevisiae with dissolvedair flotation, was investigated [12]. The obtained results were supported by the physicochemical characteristics of the surfactants used as flotation reagents.
The old mines closure all over the world, due the decrease in the ore grade, lower metal prices etc., represents an often pollution candidate problem from acid mine or rock drainage. For instance, although the underground extraction of Pb-Zn mineralization in a Tunisia’s mine stopped in 1958, a large volume of flotation tailings (more than 500 Mt) containing sulphides were deposited in a tailings impoundment [13]. The extracted ore was treated at first by gravimetry (during the period of 1901-1949) and by differential flotation (during the period of 1950-1958). Physical, chemical, and mineralogical characterization (X-ray diffraction), reflected light microscopy, scanning electron microscope of these samples were performed.
The effect of chemical coagulation and biological auto-flocculation relative to ζ-potential was studied to compare flotation and sedimentation separation processes for algae harvesting [14]. The experiments revealed that microalgae separation was related to auto-flocculation of Anabaena spp. Dissolved CO2 flotation as an alternative to dissolved air flotation was examined, too. Microalgae were significantly separated in response to anionic surfactant, as a function of bubble size and ζ-potential. Aim of this review paper is to describe the advances in research studies and activities on flotation, with main focus the contribution of physical chemistry to the process.
Surface Chemistry
The flotation behaviour of methylated quartz particles of different size, but within the size range from 0.2 to 50 μm, and varying contact angle, has been probed in a mechanical flotation cell [15].
The results suggested that particles of a given size need to possess a minimum critical contact angle for flotation to occur. The flotation behaviour of fine particles was also studied in a previous work by this Australian group [16]. It was indicated that the minimum critical contact angle increased in value as particle size decreased, for flotation to be initiated. As a consequence, a non-floating component exists within a given size fraction. The critical contact angle for flotation was explained in terms of the existence of an energy barrier for bubble-particle attachment.
Understanding the limits of fine particle flotation is the key to the selective separation of fine mineral particles. Fines have low collision efficiencies with gas bubbles and float slowly. There has been a great deal of work aimed at overcoming the inefficient collision of small particles with rising air bubbles [17].
Generally, flotation is considered to consist of three major subprocesses, namely, the collision, attachment and detachment [18]. The collision involves the approach of a particle to an air bubble in the field of flow. The collision sub-process is governed by the liquid flow and the relative motion between bubbles and particles in a flotation cell.
Many industrial processes, at some stage, generate or utilize fine particles ranging in size from over 100 μm to less than 1 μm [4]. This is usually the case in mineral processing. Ultra-fine particulate matter, however, exists in water and wastewater, too, needing removal before it is made potable or is dischargedlooking for sustainability. The availability of an effective separation technology is essential, because of the impact on downstream operations and product quality. Flotation, therefore, is one of the processes available for the separation of particulate matter from dispersions.
Hydrophobicity, in general, was indirectly measured by: (i) contact angle measurements, where the greater the contact angle, the more hydrophobic is the system, and (ii) surface tension measurements of various systems, including mineral or biological. For instance, the effect of pH on the contact angle of magnesite (MgCO3) was examined at different flotation collector concentrations [19]. The measurements were conducted, in parallel also with dolomite, according to the usual technique for flat surfaces; the air bubbles used in this study were characterized as being of medium size. It was shown that the curves of solution pH versus contact angle, in presence of sodium oleate, and pH versus magnesite flotation recovery had the same shape (Figure 1).
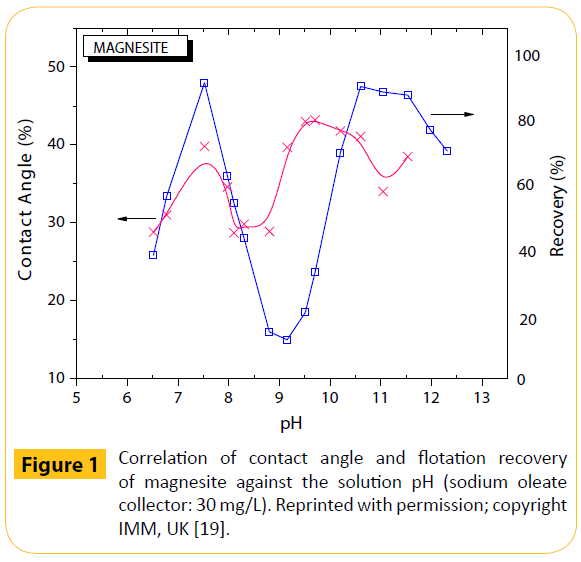
Figure 1: Correlation of contact angle and flotation recovery of magnesite against the solution pH (sodium oleate collector: 30 mg/L). Reprinted with permission; copyright IMM, UK [19].
Collector adsorption studies on the two minerals followed. Read sorption of magnesium di-oleate molecules by hydrophobic aggregation was discussed as likely to be important in the enhanced recovery, at near neutral pH. An application for nanoparticles as flotation collectors was reported-which looks like the older carrier flotation [20]. In order to assess the ability of the nanoparticles to increase the contact angle, model experiments were conducted: the cationic nanoparticles were allowed to deposit onto clean microscope slides and so, water contact angles could be easily measured.
Surface chemistry issues, as surface tension, are incorporated in several treatment methods, such as the adsorption of specific chemical agents (termed collectors) to enhance process efficiency, the change of hydrophobicity of a solid particle for the purpose of separation, that is, by the application of flotation etc. Especially, the concept of critical surface tension for the examination of wetting effects proved to be important to improve selective flotation [21].
The important role of dissolved oxygen in collector adsorption on sulphide mineral surfaces has been extensively discussed [22]. Xanthates and dithiophosphates are the most commonly used collectors. Many other collectors, anionic and cationic, have nevertheless been developed and tried for sulphide minerals. Galvanic interactions during grinding cause changes on the sulphides surface, which affect the hydrophobicity [23].
In Figure 2, the electrokinetic behaviour of both sulphides, pyrite (FeS2) and arsenopyrite (FeAs), was compared with the addition of potassium permanganate; these minerals are known to be easily oxidized [24].
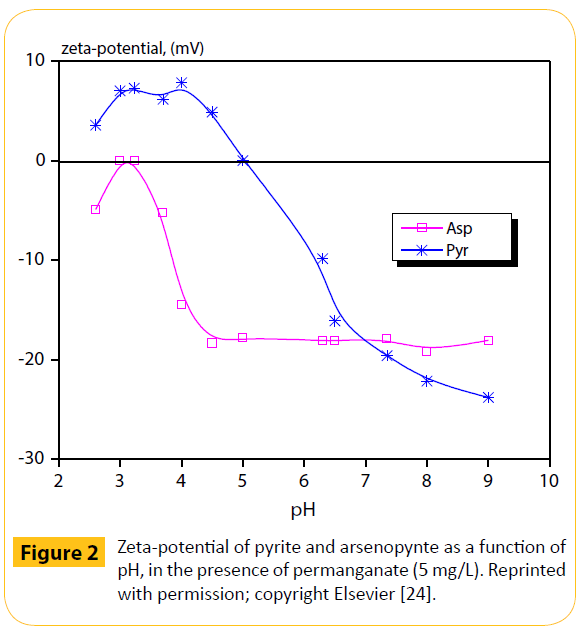
Figure 2: Zeta-potential of pyrite and arsenopynte as a function of pH, in the presence of permanganate (5 mg/L). Reprinted with permission; copyright Elsevier [24].
The products of permanganate reduction, under these conditions, are probably non-stoichiometric varieties of the Mn(IV) oxide, MnO2. An Eh-pH equilibrium diagram of manganiferous species was presented, too. Focus of this work was mainly the fact that gold is often associated with arsenopyrite, often exhibiting a direct relationship between Au content and As grade–i.e. concentrating gold.
Beattie and Poling [25] studied the surface chemistry of arsenopyrite and its depression in flotation using xanthates (i.e. O-alkyl dithiocarbonates) by oxidizing agents. The mechanism of the action of reducing reagents (as the sulphur-containing salts) during flotation in acidic media was related to the role of dissolved oxygen and the different oxidation behaviours of pyrite and arsenopyrite. Since dixanthogen is the more significant collector species in pyrites flotation, it is apparent that every chemical reagent which hinders its formation will act as a depressant of sulphide minerals. According to the decomposition reaction of dixanthogen, each chemical more reducing than the ethyl xanthate will depress flotation by not permitting its oxidation [26]. Compared to arsenopyrite, pyrite requires a lower dissolved oxygen concentration to balance the anodic oxidation of xanthate to dixanthogen, which leads to its flotation. This means that pyrite flotation is possible in a less anodic potential range than that required for the flotation of arsenopyrite. The results on an investigated industrial sample were elsewhere summarized correlating the modifier addition with redox potential values [27]. It was found that a narrow potential range (0.18-0.16 V) existed where pyrite/arsenopyrite separation seemed to be possible.
Also, toxic metals are known to exist in the effluents of many industrial operations; the impact of chemical speciation was stressed during various flotation applications for metal ions separation from the effluent [28]. Thermodynamic equilibrium diagrams and software packages were employed to interpret the removal mechanism involved.
An innovative hybrid cell was investigated that combines the advantages of both flotation and membrane separation, while overcoming their limitations and having, as an outcome, clean water from an industrial wastewater. A large number of techniques have been used today to limit the membrane fouling and among them, certainly, is air bubbling, constituting also the transport medium in flotation, as applied in wastewater treatment [29]. Ultimate scope of the latter was the application on-site of the aforementioned hybrid cell for the removal of metal cations from open-pit copper mine drainage water, in Bulgaria.
Frothers are known to play a crucial role in mineral flotation. With scarcity of fresh water resources, flotation plants are increasingly under pressure to supply their water needs from other sources such as bore water, seawater or recycled plant water. This water generally contains a high concentration of inorganic electrolytes, which may have a substantial influence on the performance of the flotation operation. So, the stabilization mechanism of bubbles by salt was investigated [30].
Particle Size and Flotation
The control of particle (and, as it will be later described, also of bubble) size is of paramount interest in affecting the process and particularly, referring to fine flotation in minerals and/or chemical engineering [31]. Froth flotation (i.e. a dispersed-air technique) is the commonly used one for the separation of valuable from gangue minerals [32]. A machine vision system was developed to characterize the process behaviour at different operating conditions. Bubble size distribution, number of bubbles, froth velocity and stability were the main visual features extracted from the froth images. A simple empirical dimensionless model was presented to calculate the mineral gangue recovered per size class by entrainment, in terms of the water recovery in an industrial flotation cell [33].
Recently, a work of single bubble-single particle interactions was reported being of interest to flotation applications [34]. An experimental device was developed where a standing bubble was approached at prescribed flow velocity by an aqueous dispersion of particles-much smaller than the bubble. Bubbles of different diameter (500-1700 μm) under different flow rate (liquid velocity in the column 0-8 mm/s) were obtained. Two separate high-speed cameras were employed to monitor the bubble surface from two different Cartesian directions, allowing thus a 3D perspective of particles trajectories and collisions with the bubble. A special feature of the device was that the velocity of the suspension and the size of the bubble could be independently adjusted in a range of values that corresponded to the flotation process. A theoretical model was then developed to describe the particle trajectories and velocities. It was concluded that the primary mechanism of collision was interception, in that case.
In another paper, the development of a general expression was sought for the flotation rate, in a combined gravitational and turbulent flow field, which covered in a unified and consistent way all possible sets of the problem parameters [35]. As, despite the significance of turbulent fluid motion for enhancing the flotation rate in several industrial processes, there had been no unified approach for the modeling of flotation rate in a turbulent flow field. This was achieved by using concepts from statistical approach to homogeneous turbulence and gas kinetic theory.
The full particle motion equation with the inclusion of inertial, gravitational and corrections for micro-hydrodynamic forces for both fully mobile (i.e. the potential flow) and immobile bubble (the Stokes flow) surfaces were developed and numerically solved to analyze the bubble-particle collision interaction [36]. The effect of particle density, size and film thickness on bubbleparticle collision angle and efficiency was examined numerically. The influence of micro-hydrodynamics on the collision angle and efficiency was found significant.
A comparison between dispersed-air, the typical method of bubbles generation and dissolved-air flotation (termed DAF), applied to metal-loaded goethite, was presented [3]. Dissolvedair flotation, being the established technique in water treatment, is quite different from dispersed-air flotation. Dissolved-air flotation is based on the varying solubility of air in water according to the pressure in the saturation tank, i.e. following Henry’s law, and applying actually the idea of a recycle reactor. The (above mentioned) two techniques are essentially different with regards, for instance, the bubbles size and flow conditions. In DAF processes micro-bubbles of 10-80 μm in size are generated.
The results for zinc removal are given as Figure 3. The air recycle was the main parameter studied (at least 20% was necessary) for dissolved-air flotation.
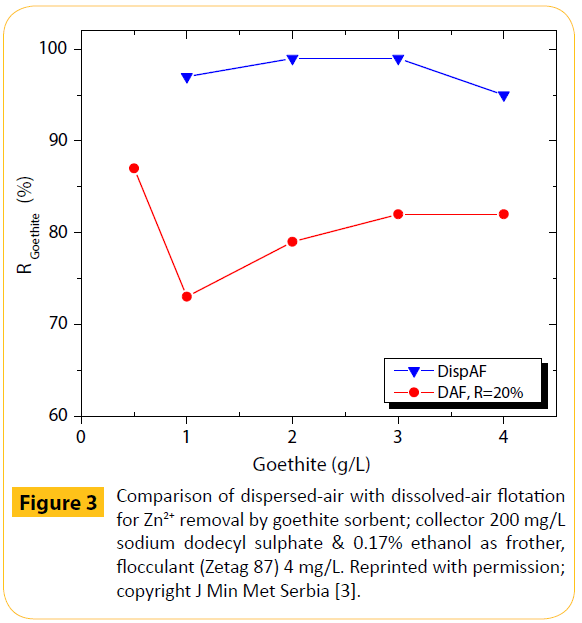
Figure 3: Comparison of dispersed-air with dissolved-air flotation for Zn2+ removal by goethite sorbent; collector 200 mg/L sodium dodecyl sulphate & 0.17% ethanol as frother, flocculant (Zetag 87) 4 mg/L. Reprinted with permission; copyright J Min Met Serbia [3].
A flocculent was also required, particularly for DAF. Generally, both flotation techniques gave good flotation recoveries. Nevertheless, the dispersed-air flotation looked preferable. Meanwhile, the metal removals were near 100%. The heavy metals recovery by flotation followed a mechanism such as precipitation, adsorption or complexation, for the effective metal ions removal.
A computational fluid dynamics (CFD) model incorporating flotation kinetic expressions was elsewhere developed to simulate the performance of flotation tanks utilized in water and wastewater treatment plants [37]. The focus here was on tanks that operate without any external means of flow mixing (impeller, etc.), such as DAF tanks, where buoyancy, particle settling, bubble and turbulence are mechanisms contributing comparably to local flotation rates.
Alternative Flotation Methods
It was earlier said that although much attention has been paid to the particle size, the role of bubble size was somehow comparatively disregarded in the litereature, hence deserved more attention [31]. The explanation given for the former was the fact that there are mainly practical reasons in the bubble size change. Nevertheless, various bubble generation methods exist, certain unconventional, which utilize fine bubbles.
Flotation experiments were carried out in a cell in which bubbles of known size could be generated independently of the turbulence levels, which could be controlled by varying the impeller speed; the mean bubble size ranged from 75 to 655 μm [38]. Results were reported for the flotation rates of fine particles (polystyrene latex, quartz and zircon.), of less than 50 μm diameter. Frothers, which are used in flotation to reduce bubble size, have as a consequence to increase gas holdup. The advantage of gas holdup is that it is easy to determine in two-phase (air-water) systems by measuring pressure difference over a known distance [39].
As an important indicator of flotation performance, bubble structure is believed to be related with the addition amount of chemical reagent. To differentiate film size calculated from surface froth image segmentation and underlying bubble size, postsegmentation analysis was carried out to archive the prediction of bubble size distribution from the known bubble film distribution [40]. The bubble surface area flux (defined as the ratio between the superficial gas rate and the Sauter mean bubble diameter) has been also used to describe the gas phase dispersion efficiency in flotation machines [41].
A correlation was published, based on experimental data (from the literature), between the critical coalescence concentration and hydrophilic-lipophilic balance/molecular weight ratio for surfactants used as flotation frothers [42]. The usage of biosurfactants as potential alternatives to chemosynthetic surfactants in controlling bubble behaviour in the flotation process was examined elsewhere [43].
It was argued that the problem of processing mineral fine particles poses an immense challenge, due to the relative costs of conventional methods when applied in that case; so, the flotation techniques of dissolved-air flotation and electroflotation were proposed and described, during a scientific meeting with the remarkable title of “Mineral Processing at a Crossroads”-at least for Europe [44]. The former method was reviewed for its water treatment application [45].
Figure 4 presents a comparison between the two flotation techniques, dispersed-air and dissolved-air; the metal analysis was undertaken following flotation [46]. Dispersed-air flotation produced higher removals for all the examined metals, compared with dissolved-air flotation. The reason for this was said to be the fact that in the dispersed-air technique unit, the hydrodynamics of the complex solid/liquid/air system were more intensive, presumably due to the existence of air bubbles having larger diameters, as well as to the greater number of them.
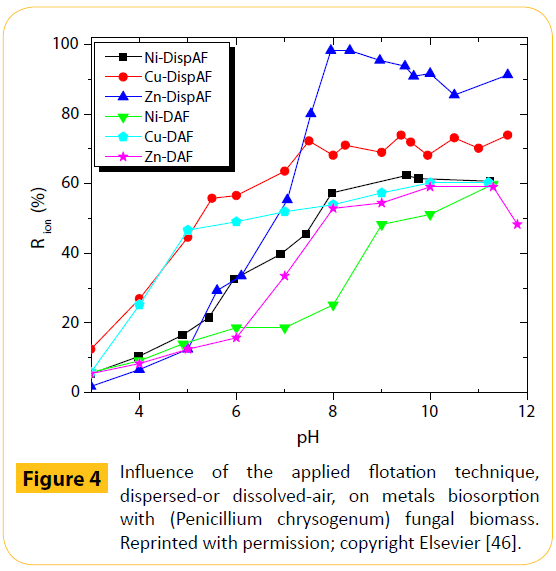
Figure 4: Influence of the applied flotation technique, dispersed-or dissolved-air, on metals biosorption with (Penicillium chrysogenum) fungal biomass. Reprinted with permission; copyright Elsevier [46].
Therefore, the resultant highly turbulent mixing conditions favoured biosorption. The metal ions separation from environmental sources by sorptive flotation has been recently reviewed in-depth [6].
The process of biosorption, applied for the effective removal (or recovery) of dissolved metals from aqueous waste streams is based upon several mechanisms, the most important of them being physical adsorption (electrostatic forces), ion exchange, surface complexation and surface precipitation [47]. Microbial metal uptake by non-living cells, which is metabolism-independent passive binding (adsorption) to cell walls, as well as to other (usually fragmented) external surfaces, is generally considered as a rapid process, taking place within a few minutes [48]. Biosorptive flotation of dilute copper solution was also carried out [49]. Centrifugation and sedimentation, the conventional separation techniques, were suggested as not being suitable to be employed in the case of metalbearing biosorbent due to the small size of solid particles present in the solution. The by-products, sludge and wastes obtained from food, beverage, dairy and agricultural industries are an abundant source of biosorbent.
A recent work presented results showing (measuring) the limits of dissolved-air flotation as a function of particle size distribution, solids content and air superficial velocity [50]. Interestingly, the microbubbles were found to be not selective with respect to particle size, floating both fine and coarse particles, which is most likely due to the existence of several mechanisms acting on the flotation of particles by these minute bubbles.
Certain results from a conducted comparison between dispersed-air and electroflotation were discussed (see, for example Figure 5), then proposing further research of the later. The great difference, particularly of the magnesite behaviour, was thought to be due to the existence of ultrafines on magnesite particles; this salt-type mineral was of cryptocrystalline (amorphous) nature and very fine particles were even created during conditioning, by the stirring action of impellers [51].
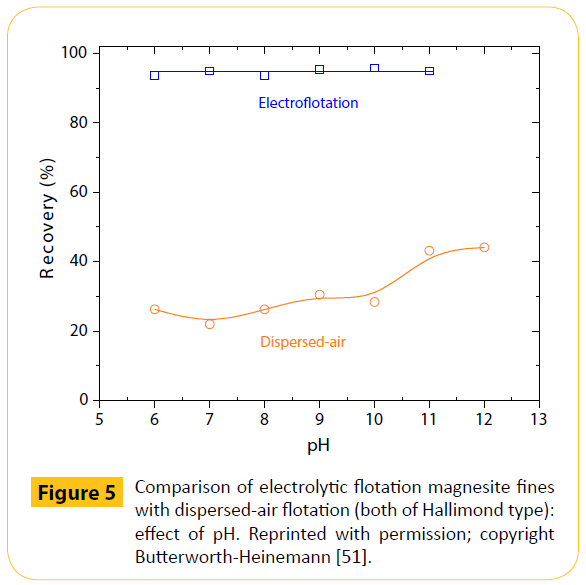
Figure 5: Comparison of electrolytic flotation magnesite fines with dispersed-air flotation (both of Hallimond type): effect of pH. Reprinted with permission; copyright Butterworth-Heinemann [51].
Electroflotation (or electrolytic flotation) is an unconventional separation process owing its name to the bubbles generation method it uses, i.e., electrolysis of the aqueous medium.
It was concluded that although froth flotation is a commonly applied selective separation process in mineral processing, it becomes inefficient for beneficiating fines. The size of the produced bubbles by electroflotation was measured to have a mean diameter of 50 μm [52]. A feature of electroflotation is the ability to create very fine bubbles, which are known to improve flotation performance of fine particles [53]. Electroflotation offers certain advantages over other flotation techniques of bubble generation, such as the dispersed-air or dissolvedair. Among others, the following points are perhaps the most important [5]: (i) the electrode grids can be arranged to provide good coverage of the whole surface area of the flotation tank, avoiding any by-pass, as shown by a hydrodynamics investigation; (ii) the electric field gradient between electrodes aids flocculation and flotation, even without the presence of any surfactant; (iii) gas production (evolution), flotation time, and the other operating conditions can be checked quickly and are relatively easily controlled; (iv) the equipment is reliable and safe in operation; (v) Ionic strength of the effluent solution (slurry) to be treated is not a crucial parameter, as in the other flotation techniques.
Figure 6 presents a comparison for pyrite anionic flotation [54]. An increase of the permanganate (a strong oxidizing reagent) concentration increased the dispersed-air flotation of pyrite. In electrolytic flotation, however, a different behaviour was observed, with an increase of permanganate up to 10 mg/L (with around 85% recovery). While further increase of permanganate depressed pyrite, to such a point that practically no flotation was observed. At the same time, the zeta-potential of pyrite did not change appreciably. It was clear, from the long inserted discussion, that the oxidation of the pyrite surface played a decisive role in its flotation behaviour.
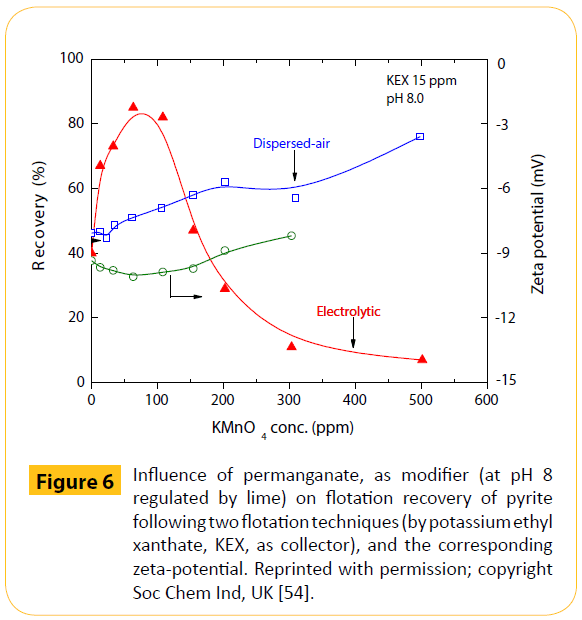
Figure 6: Influence of permanganate, as modifier (at pH 8 regulated by lime) on flotation recovery of pyrite following two flotation techniques (by potassium ethyl xanthate, KEX, as collector), and the corresponding zeta-potential. Reprinted with permission; copyright Soc Chem Ind, UK [54].
In order to explain this difference in flotation (by ethyl xanthate) behaviour, thermodynamic calculations for the system Fe-EX-H2O have been done. Electrolytic flotation was affected strongly by the flocculation of particulates, which was pronounced in lowcharged particles.
Conclusion
As it was already referred, flotation initially originated from the field of mineral processing. The importance of this process to the economy of the whole industrial world is considered to be enormous. Hopefully it was shown in the aforementioned that basic chemistry has been (and can be) used to explore well flotation–i.e. possibility if it works in a system, expectation of the result/recovery, mechanism of this separation process itself, explanations etc. Certain significant activities in flotation research were reviewed in the present, with main focus in the contribution of physical chemistry to flotation; i.e. zeta-potential measurements, contact angle etc., including examining the role of bubble and particle size played with regard to the process. Some typical examples from the literature were briefly presented, as far as both mineral processing and also water treatment are concerned. By-product recovery is a potentially profitable aspect of flotation treatment, so proteins, fats, oil, organics, but also toxic or valuable metals could be recovered from waste streams, especially when no harmful chemical reagents are added to help the pollutant separation. Alternative bubble generation methods were examined, such as electroflotation and dissolvedair flotation-the latter technique is mainly intended for water treatment applications.
Conflict of Interest
The authors declare no conflict of interest.