Keywords: Plant protease inhibitors; COVID-19; serpins; antiviral; therapeutics
Key Highlights
- Plants are one of the most important and natural source of protease inhibitors (PIs).
- In plants, PIs are known to act in the defence mechanism against pathogens.
- Plant PIs have been known to possess antiviral activities against several pathogenic viruses such as HIV, Hepatitis C virus and human cytomegalovirus (HCMV).
- Plant PIs can inhibit the main protease (Mpro or 3CL) of SARS-CoV-2 essential for processing of the polyproteins of the virus into functional proteins.
- Plant PIs also act as inhibitory molecules against TMPRSS2, a transmembrane protein present on the host cell, required by the virus to enter into the host cell.
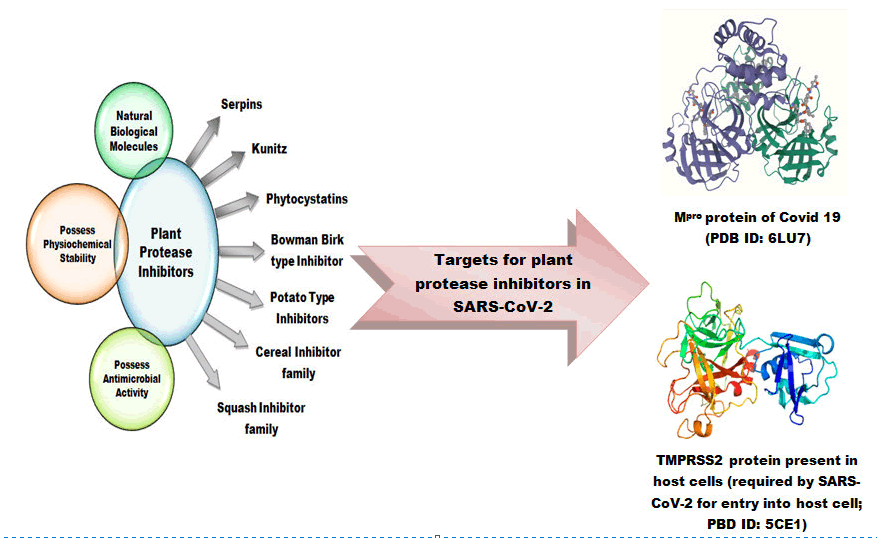
Graphical Abstract
Introduction
Proteases (peptidases or proteinases) are one of the most important hydrolytic enzymes with discernable roles in several physiological and biochemical processes. Even though these enzymes are highly essential for maintenance and survival of their host as they are involved in signal transduction, protein-protein interaction, inflammatory response, protein catabolism, blood coagulation and digestion, their regulation is very crucial as they as they can be potentially harmful (Clemente et al., 2019; Fan et al., 2020).
Protease inhibitors (PIs) are highly active compounds which are involved in important physiological reactions related to metabolism, cell physiology and regulation of proteolytic action. In a number of biological pursuits like blood clotting, apoptosis, hormone processing and inflammation, the PIs are now treated as very important signaling molecules (Srikanth and Chen, 2016). They are widely distributed in plants and animals. In plants, PIs are present as small proteins in the storage tissues such as seeds and tubers in high concentrations and in other tissues they exist in low concentrations. Plant PIs act as storage proteins in the form of nitrogen sources, also are involved in modulation of enzymatic processes, regulation of apoptosis and defence mechanism against animals, insects and microorganisms (Volpicella et al., 2011). Plant PIs possess a notable resistance to heat treatment and a high stability against alterations in ionic strength, pH, proteolysis as well as denaturing agents due of the high content of cysteine residues in disulfide bridges (Cotabarren et al., 2019). Several recent investigations report novel biologic activities for plant PIs such as antimicrobial activities, anticoagulant as well as antioxidant action plus inhibition of tumor-cell growth; thus marking their significance in medicine as effective tools for inactivating proteases involved in several human diseases like arthritis, pancreatitis, thrombosis, emphysema, hypertension, cardiovascular morbidities, neurodegenerative diseases (such as Alzheimer’s disease) and muscular dystrophy. They have been employed in several fields of biotechnology and agriculture and the spread of several pathogens that cause life threatening diseases like cancer, AIDS, hepatitis, malaria and various others have proved to be prevented by using plant PIs in drug design (Cotabarren et al., 2019). In order to be used in humans, the PIs should be capable of inhibiting each of the major intestinal proteases, such as pancreatic trypsin, α-chymotrypsin, as well as elastase and must be potentially nontoxic. PIs are being commercially used for deterrence of protease-induced perianal dermatitis and several non toxic PIs have been isolated and purified from barley seeds, cabbage leaves, and Streptomyces (Kim et al., 2009).
PIs are found in plants belonging to a variety of systematic groups especially the plants belonging to the Solanaceae family harbour several high levels of PIs. (Kim et al., 2009). In plants PIs were first discovered as chymotrypsin and trypsin inhibitors in tomatoes infected with Phytophthora infestans and were correlated to plant resistance to pathogens (Wolosluk et al., 1991). Later serine PIs of 20-24 kDa were found in potato tubers in response to infection with P. infestans and mechanical wounding (Valueva et al., 1998, 2003).
Classification of Plant Protease Inhibitors
This On the basis of primary and tertiary structure, including the number and position of disulphide bonds and active sites, PIs can be classified in four groups according to the class of proteases they inhibit: serine protease, cysteine protease, metallocarboxy-protease or aspartic protease (Laskowski and Kato, 1980). Based on structural and biochemical properties, plant PIs have also been classified as serpins and Bowman-Birk serine (BBIs), cysteine, potato type I and type II PIs, cereal trypsin/α-amylase, mustard trypsin, squash inhibitors, metallocarboxypeptidase and soybean trypsin (Kunitz) inhibitors (Table 1). On the basis of their amino acid similarities and the structures obtained the 48 identified plant PIs are grouped into 26 related superfamilies’ (or clans). According to the MEROPS database, the inhibitors have 82 family members (Fan et al., 2020). Different classes of plant PIs exhibit different mechanisms through which they interact with the target proteases. Some of the PIs utilize an irreversible inhibition of proteolytic activity (e.g. serpins) while most of them exhibit a canonical-competitive inhibition mode via ‘substrate-like’ binding to the catalytic domain of the targeted protease (e.g., BBIs and Kunitz inhibitors) or they make use of a non-catalytically competitive inhibition (e.g. cystatins or mustard-type PI) else they may act via a mixed mode, where the primary competitive binding to the active site is supported by a secondary binding event (e.g. metalloprotease inhibitors; Hellinger and Gruber, 2019)
Serine PIs or serpins constitute the major class of plant PIs, which have been classified into more than 20 families. Serpins are mainly found in plants belonging to the Solanaceae, Fabaceae, Euphorbiaceae, Poaceae, and Cucurbitaceae families (Haq et al., 2004; Fan and Wu, 2005) with most of these being isolated from barley grain, wheat grain, rye, wild oats, pumpkin, and A. thaliana (Volpicella et al., 2011). In plants they are responsible for controling protein synthesis and turnover, and physiological functions such as fertilization, growth and development, digestion, cell signaling or migration, immune defense, wound healing and disease propagation. They play crucial role in the pathogenesis and/or host tissue penetration of a number of diseases, such as cardiopulmonary disease and emphysema (Mishra et al., 2020). Serpins display a distinctive mechanism of irreversible inhibition termed as “suicide substrate” mechanism rather than the standard reversible inhibition mechanism used by other PIs. They are metastable proteins with a molecular weight usually higher than 40 kDa (Patson and Gettins, 1996).
A report demonstrated that in serine PIs, the ‘reactive sites’ are mutating faster than amino acids in the rest of the proteins, implying that their roles in defense against microorganisms (and insects) may exert a strong selection pressure on these proteins to conserve the reactive sites and that this selection may be related to plant defense (Ryan, 1990). Serine PIs also called serpins inhibit both serine and cysteine proteases (Cohen et al., 2019). Although several serpins with inhibitory activity against caspases and papain like cysteine proteases have been reported but they predominantly act against trypsin like serine proteinases (Jamal et al., 2013).
Another important class of PIs is the inhibitors of the cysteine proteases (cystatins or phytocustatins) which range in molecular weight from 10 kDa to 23 kDa. They inhibit cysteine proteases in a non-catalytically competent manner (i.e. although they do not bind to proteses in a strictly substrate-like manner but they still block access to the catalytic site; (Hellinger and Gruber, 2019). Cystatins regulate endogenous and heterologous cysteine proteases in a variety of physiological processes such as abiotic stress tolerance, protection against insects and nematodes via inhibition of digestive enzymes in their gut, regulation of peptidase activity during apoptosis, protection of cytosolic metabolism from intracellular peptidases released by incidental rupturing of protein bodies. They have been isolated and characterized from a number of vegetables and crop plants such as cabbage, apple, papaya, avocado, carrot, cowpea, ambrosia, castano, seeds of wheat, maize, sunflower, soybean, sugarcane, rice etc (Cotabarren et al., 2019).
The Kunitz and BBI have been observed in the leguminous family and they generally range in size from 18-24 and 5-16 kDa, respectively. Both of them function via competitive inhibition of protease using the standard mechanism of substrate like binding to the catalytic site of the protease. Kuntiz inhibitors are known to function in the regulation of physiological homeostasis and in inhibition of pathogenic proteases while the expression of BBIs in plants is strongly induced by pathogenic invasion (Hellinger and Gruger, 2019). Other than this a few aspartate and metalloprotease inhibitors have been reported which are isolated from potato tubers, sunflower flowers, barley and thistle (Cynara cardunculus). Metalloprotease are highly compact and stable proteins in nature because of the high content of disulphide bonds in them (Cotabarren et al., 2019).
Antiviral Potential of Plant PIs
Oral According to various reports, serine PIs in plants provide protection against various pests and pathogens. In most of the pathogenic organisms like bacteria, fungi, viruses, insects and vertebrates, proteases comprise around 1-5% of the genome among which majority of the functions are performed by serine proteases (Mishra et al., 2020). The NS3 protein of Hepatitis C virus (HCV) is a chymotrypsin like protein which contains a serine protease domain that is responsible for processing of the HCV polyprotein as well as the active site of the Human cytomegalovirus (HCMV) contains a Ser-His-His catalytic triad and therefore is a serine protease which is essential for capsid formation during viral replication (Bianchi and Pessi, 2002; Fischmann and Weber, 2002). Therefore, serpins can be effectively utilized to attenuate such serine proteases thereby providing protection against a wide variety of pathogens. Novel antiviral strategies include targeting either host or viral accessory protein to ultimately block viral replication or inhibit cellular proteins necessary for the virus life cycle. Proteolytic cleavage of the precursor hemagglutinin (HA0) into HA1 and HA2 subunits by host proteases is essential for fusion of HA with the endosomal membrane and thus represents an essential step for viral infection (Mishra et al., 2020). The trypsin PIs from the leaf extract of Capsicum baccatum var. pendulum inoculated with Pepper yellow mosaic virus (PepYMV) significantly reduced the yellow mosaic viral infection (Moulin et al., 2014). The Cucumis metuliferus serine PIs (CmSPI) gene when overexpressed and silenced in Nicotiana benthamiana and Cucumis metuliferus displayed potyvirus resistance and synchronous development of potato ring spot viral symptoms respectively (Mishra et al., 2020). The sunflower trypsin inhibitor (TI) from Helianthus annuus is the smallest known BBI which has been explored as a model peptide for drug design (Craik, 2009; Craik et al., 2013; Elliott et al., 2014). Various plant PIs displaying antiviral activity have been previously reported (Table 2).
The Kunitz trypsin inhibitors isolated from B. variegate and G. max seeds termed BvvTI and KBTI respectively, display significant activity against the HIV-1 reverse transcriptase as well as also possesses anti-tumor activity against the human nasopharyngeal cancer cells, human breast cancer cells and hepatoma cells (Fang et al., 2010a; Fang et al., 2010 b) . Another Kunitz trypsin inhibitor, BSKT1 isolated from G. max cv. dull black seeds also possess anti HIV-1 reverse transcriptase activity (Lin and Ng, 2008). According to the invention of Domagala et al., (1996) the derivatives of coumarin which is found in fruits (bilberry and cloudberry), green tea, chicory, soy, higher plants such as Rutaceae and Umbelliferone as well as the stem bark of Calophyllum dispar (Clusiaceae) are inhibitors of aspartyl proteases, especially the aspartyl proteases of retroviruses such as HIV and hence can be expected to be used as an antiviral agent in the treatment of retroviral infections. They also have been found to be potential therapeutics for treatment of malaria, mycoplasmosis, Q fever and mononucleosis (Domagala et al., 1996; Jain and Joshi, 2012). Ye and Ng in 2002 isolated a novel trypsin chymotrypsin inhibitor from Vicia faba (commonly known as bakla in India) seeds which displayed anti HIV-1 reverse transcriptase activity as well as antifungal activity against Mycosphaerella arachidicola and Physalospora piricola (Ye and Ng, 2002). A novel, fairly stable Kunitz trypsin inhibitor of serpin family was isolated from Allium sativum (garlic) by Shamsi and colleagues which could act as a potential nontoxic therapeutic against a number of viral diseases (Shamsi et al., 2016).
| Family |
Protease Inhibitor |
Plant source |
Characteristic |
Reference |
| Serpins |
At-serpin 1 |
Arabidopsis thaliana |
Acts against metacaspase in vivo and plays role in plant immunity |
Vercammen et al., 2006 |
|
OSZa-d |
Oat (Avena sativa L.) grain |
OSZa and OSZb are |
(Hejgaard and Hauge 2002) |
|
|
|
efficient inhibitors of pancreatic elastase. |
|
|
|
|
OSZb is an inhibitor of chymotrypsin whereas OSZc is a fast inhibitor of trypsin. |
|
|
|
|
Together they display a broader activity against the digestive serine proteinases than the other serpins from rye, barley or wheat. |
|
|
CmPS-1 |
Cucurbita maxima |
Possess anti-elastase activity. |
Yoo et al., 2000; Jamal et al., 2013 |
|
(Cucurbita maxima phloem |
|
May impart resistance against bacteria, insects and phytoplasma. |
|
|
serpin-1) |
|
|
|
|
WSZI |
Triticum aestivum |
Inhibits chymotrypsin and Cathepsin G |
Hellinger and Gruber, 2019 |
|
HorvuZx (BSZx) |
Hordeum vulgare |
Inhibits trypsin, chymotrypsin, Factor Xa, thrombin, Factor VIIa, plasma kallikarein and leukocyte elastase. |
Hellinger and Gruber, 2019 |
| Kunitz |
Kunitz trypsin inhibitor |
Artocarpus |
Inhibits elastase, trypsin and chymotrypsin. |
Bhat |
|
|
Integrifolia (Jackfruit) |
|
and Pattabiraman, 1989 |
|
|
|
However it displays a very poor action on Streptomyces |
|
|
|
|
caespitosus and Aspergillus oryzae proteases |
|
|
Tamarind Kunitz inhibitor |
Tamarindus indica |
Inhibits trypsin and Factor Xa |
Hellinger and Gruber, 2019 |
|
|
Glycine max |
Inhibits plasmin, human Fcator XIIa, plasmin kallikrein, trypsin, chymoreypsin |
Hellinger and Gruber, 2019 |
|
SKTI-3 |
|
|
|
|
PdKI-2 |
Pithecelobium |
Inhibits trypsin as well as papain, a cystein protease. Active against digestive enzyme of larvae from |
Oliveira et al., 2007 |
|
|
dumosum seed |
diverse orders and hence can be used as a potent insect |
|
|
|
|
antifeedant |
|
|
Kunitz inhibitor CPTI |
Cicer arietinum |
Show differential inhibitory activity against |
Harsulkar et al., 1999 |
|
|
|
trypsin, chymotrypsin, H. armigera gut proteases |
|
|
|
|
and bacterial protease(s) |
|
|
PFTI |
Plathymenia |
Inhibits bovine trypsin and bovine chymotrypsin. |
Silveira |
|
|
foliolosa |
|
et al., 2008 |
|
|
|
Exhibits |
|
|
|
|
significant inhibitory activity against on larval midgut |
|
|
|
|
proteases of A. kuehniella and D. saccharalis |
|
|
PCP1 6.6 and PCPI 8.3 |
Solanum tuberosum |
Possesses inhibitory action against cathepsin B, H and L. |
Hellinger and Gruber, 2019 |
|
|
|
|
|
|
|
|
Also inhibits dipeptidyl peptidase I and Clostripain. |
|
| Bowman birk type inhibitors (BBI) |
Soyabean BBI (Isotype 2-II; ̫́8 kDa) |
G. max |
Inhibits trypsin, chymotrypsin, mast cell chymase, cathepsin G, matriptase, leukocyte elastase, duadenase |
Hellinger and Gruber, 2019 |
|
BTCI |
Vigna unguiculata (black eyed pea) |
Trypsin/chymotrypsin inhibitor. |
Franco et al., |
|
|
|
|
2003 |
|
|
|
Moderately active against the digestive chymotrypsin of adult insects |
|
|
AsPIs |
Acacia Senegal seeds |
Highly active against serine proteases. Possess remarkable inhibitory activity towards |
Babu and Subrahmanyam 2010 |
|
|
|
total gut proteolytic enzymes followed by trypsin and |
|
|
|
|
chymotrypsin and retards the growth and development |
|
|
|
|
of H. armigera |
|
|
BI-I (seven isotypes I-VII) |
Ananas comosus |
Possesses inhibitory activity against trypsin, papain, bormelain, cathepsin L and chymotrypsin |
Hellinger and Gruber, 2019 |
| Phytocystatin |
Oryzacystatin I and Oryzacystatin II |
Oryza sativa |
Inhibits cathepsin B, Hand L and Legumain |
Nagata, 2000; Valadares, 2010 |
|
SQAPI |
Cucurbita maxima |
Inhibits pepsin proteases |
Headey et al., 2010 |
|
Corn cystatin-I |
Zea mays |
Inhibits Cathepsin H and L |
Abe et al., 1994 |
| Potato inhibitor family |
CI-1 |
H. vulgare |
Inhibitor of trypsin, chymotrypsin, subtilin and neutrophil elastase |
Polya, 2003 |
|
PSI- 1.1 |
Capsicum annuum |
Trypsin and chymotrypsin inhibitor |
Hellinger and Gruber, 2019 |
|
TI-II |
Solanum lycopersicum |
Inhibitor of trypsin, chymotrypsin and subtilisin |
Barrette et al., 2003 |
|
PI-2 |
S. tuberosum |
Trypsin and chymotrypsin inhibitor |
Hellinger and Gruber, 2019 |
| Cereal inhibitor and squash inhibitor family |
Corn Hageman factor inhibitor |
Z. mays |
Inhibitors of serine proteases and α amylase |
Mahoney et al., 1984 |
|
|
|
|
|
|
RATI |
Eleusine coracana (ragi) |
Inhibitors of serine proteases and α amylase |
Shivraj and Pattabiraman |
|
|
|
|
1981 |
|
BTI-CMe |
H. vulgare (barley) |
Trypsin inhibitor; |
Jamal et al., 2013 |
|
|
|
Exhibits in vitro inhibition of trypsin-like proteases of the gut extracts of |
|
|
|
|
the fall armyworm, Spodoptera frugiperda (Lepidoptera: |
|
|
|
|
Noctuidae). |
|
Table 1: Plant protease inhibitors of different families.
| Protease inhibitor |
Plant source |
Antiviral activity |
Reference |
| Capsicum baccatum trypsin protease inhibitor |
Capsicum baccatum var. Pendulum |
Reduction in the yellow mosaic virus infection of Capsicum baccatum |
Moulin et al., 2014 |
| BSKTI (Kunitz trypsin protease inhibitor) |
G. max cv.Dull Black seeds |
Anti HIV-1 reverse transcriptase activity |
Lin and Ng, 2008 |
| BvvTI (Kunitz trypsin protease inhibitor) |
B. variegate seeds |
Anti HIV-1 reverse transcriptase activity as well as antitumor activity against human nasopharyngeal cells |
Fang et al., 2010 (a) |
| KBTI (Kunitz trypsin protease inhibitor) |
G. max seeds |
Anti HIV-1 reverse transcriptase activity as well as antitumor activity against human nasopharyngeal cells, breast cancer cells and hepatoma cells |
Fang et al., 2010 (b) |
| Coumarin derivatives (aspartyl protease inhibitor) |
Fruits such as (bilberry, cloudberry), green tea, chicory, soy, higher plants such as Rutaceae and Umbelliferone, stem bark of Calophyllum dispar (Clusiaceae) |
Act against aspartyl proteases of reteroviruses such as HIV. Potential therapeutic for malaria, Q fever, mycoplasmosis, nucleoplasmosi |
Domagala et al., 1996; Jain and Joshi, 2012 |
| CmSPI (Cucumis metuliferus serine protease inhibitor) |
Cucumis metuliferus |
Overexpression of the gene provides resistance to potyvirus in Nicotiana benthamiana; Silencing of the CmSPI gene in Cucumis metuliferus results in development of potato ring spot viral symptoms |
Mishra et al., 2020 |
| Novel trypsin-chymotrypsin inhibtor |
Vicia faba (bakla) seeds |
Anti HIV-1 reverse transcriptase activity as well as antifungal activity against Mycosphaerella arachidicola and Physalospora piricola. |
Ye and Ng, 2002 |
| Chymotrypsin inhibitor |
Acacia confusa seeds |
Anti HIV-1 reverse transcriptase activity |
Lam and Ng, 2010 |
Table 2: Plant protease inhibitors with antiviral activity.
The COVID-19 pandemic
In December 2019, the city of Wuhan, the capital of Hubei province in China, reported the outbreak of a pulmonary disease caused by a novel strain of coronavirus and since then the virus spread globally (Wang et al., 2020). The spread of 2019-nCoV, now officially known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still progressing despite of the severe containment measures being taken (Devaux et al., 2020). The virus consists of an RNA genome which is 82% identical to the SARS coronavirus (SARS-CoV) and both viruses belong to clade b of the genus Betacoronavirus and hence it has been named as SARS-CoV-2 and the disease caused by SARS-CoV-2 is called COVID-19 (Zhou et al., 2020; Wu et al., 2020). Although less is known about the origin of the virus but on the basis of the sequence of the viral genome and the evolutionary analysis, bats have been suspected as their natural hosts and it has been supposed that in humans SARS-CoV-2 might have been transmitted from bats via some unknown intermediate host (Guo et al., 2020). Within humans, the disease is transmitted by inhalation or contact with infected droplets released by an infected person and the incubation period ranges from 2 to 14 d. The symptoms usually consist of fever, cough, sore throat, breathlessness, fatigue, malaise etc. Although the disease is mild in most people; but in some (usually the elderly and those with comorbidities), it may advance to pneumonia, acute respiratory distress syndrome (ARDS) and multi organ dysfunction. Many people are asymptomatic. The case fatality rate is estimated to range from 2 to 3%. It was listed as a potential global health emergency by WHO due to high mortality, high basic reproduction number and lack of clinically approved drugs and vaccines for COVID-19. India too has reported more than 75,000 of coronavirus cases along with 2,440 deaths all over the country till 14th of May, 2020.
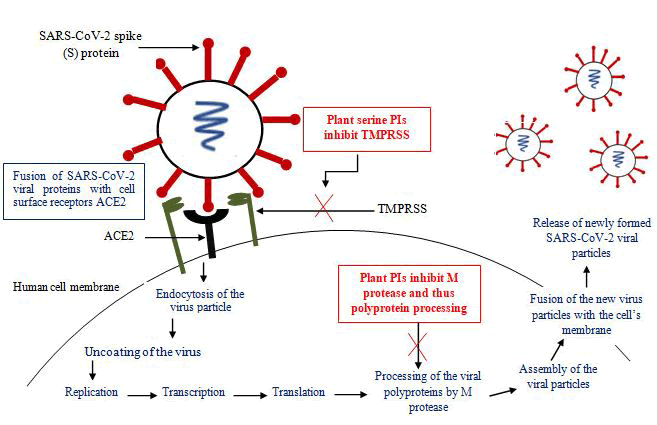
The replication cycle of the SARS-CoV-2 virus has been illustrated (Figure 1) to focus on therapeutics for efficient neutralization of virus or inhibition of some intervening virus adsorption or replication step(s). For entry into the host cell the viral S protein binds to the host cellular receptor angiotensin converting enzyme 2 (ACE2). The binding requires the host cell surface associated trans-membrane protease serine 2 (TMPRSS) for cleavage of the trimeric S protein (Guo et al., 2020). After binding of the S protein and ACE2 there occurs a conformational change in the S protein which facilitates the fusion of the viral envelope with the host cell’s membrane through endosomal pathway. After entry into the host cell, the virus un-coats itself and releases its RNA, which is replicated and translates into viral replicase polyproteins. The polyproteins are then processed into functional proteins by the main protease of SARS-CoV-2, Mpro also called as 3CL protease. The viral proteins and the genomic RNA subsequently assemble into virions in the endoplasmic reticulum and Golgi and subsequently released out of the cell (Shereen et al., 2020).
Possible inhibitory effects of plant PIs on SARS CoV-2
Most of the nation’s world-wide have been diverting their best efforts for the implementation of appropriate preventive and control strategies. Neither vaccines nor direct-acting antiviral drugs are available for the treatment of human and animal coronavirus infections (Dhamam et al., 2020). The inhibition of viral proteases necessary for proteolytic processing of polyproteins has been a successful strategy in the pharmacological treatment of HIV and HCV respectively, proving the potential of PIs for the treatment of viral infections. Similarly, the main protease of SARS-CoV-2, Mpro or 3CL is thought to be essential for viral replication and therefore, is regarded as promising target for plant PIs and antiviral pharmacotherapy (Figure 1; Fischer et al., 2020). Inhibiting the activity of this enzyme would block viral replication. Since no human proteases with similar cleavage specificity are known, inhibitors are unlikely to be toxic. Approved PIs including disulfiram, lopinavir and ritonavir have been reported to be active against SARS and MERS. Disulfiram, an approved drug to treat alcohol dependence, has been reported to inhibit the papain-like protease of MERS and SARS in cell cultures, but clinical evidence is lacking. According to the observation of Baden and colleagues lopinavir–ritonavir combination does not seem to be highly effective in patients with COVID-19 (Baden et al., 2020) and adverse gastrointestinal effects were seen in approximately 13% of the patients (Cao et al., 2020). Since better effective therapies for COVID-19 is the demand of the moment and plant PIs may prove to be potential therapeutic agents by inhibiting this main protease of the virus.
As described before, TMPRSSs plays a major role in 2019-nCoV infection as it is the main protease and allow the fusion of the virus particle with human cells. Hence, because TMPRSSs is required by the COVID-19 virus to enter into the human cells, the inhibition of this protease by nontoxic plant serine PIs may prove to be potential treatment options in 2019-nCoV infection (Figure 1; Meng et al., 2020).
Figure 1: Schematic representation of replication cycle of the SARS-CoV-2 and the possible inhibitory effects of plant PIs on its replication in human cells.
Conclusion
In conclusion, the review suggests that PIs are widely distributed in several plants where they play an important role in providing defense against pathogenic diseases. The plant PIs have been classified into different families on the basis of their structural similarity and protease inhibited. Because of their non toxic nature and fairly good stability, they have been employed in several biotechnological and pharmaceutical applications. The PIs are effective tools in inhibiting proteases associated with a number of diseases and are also highly efficient in inhibiting viral proteases, they can be employed as a potential therapeutic in the treatment of the ongoing COVID-19 pandemic which has been declared by the WHO as a global emergency. Further docking and in vivo studies are required for finding the possible use of these plant PIs in the treatment of COVID-19.
References
- Abe M, Abe K, Iwabuchi K, Domoto C, Arai S. (1994). Corn cystatin I expressed in Escherichia coli: investigation of its inhibitory profile and occurrence in corn kernels. J Biochem; 116:488
- Babu SR and Subrahmanyam B (2010). Bio-potency of serine proteinase inhibitors from Acacia senegal seeds on digestive proteinases, larval growth and development of Helicoverpa armigera (Hủ̬bner). Pesticide Biochem Physiol
- Baden LR and Rubin EJ (2020). Covid-19 The search for effective therapy. N Engl J Med
- Barrette-Ng IH, Ng KKS, Cherney MMâ?á, Pearce G, Ghani U, Ryan CA and James MNG et al. (2003). Unbound form of tomato inhibitor-II reveals inter-domain flexibility and conformational variability in the reactive site loops. J Biol Chem; 278: 31391.
- Bhat AV and Pattabiraman PN (1989). Protease inhibitors from jackfruit seeds. J Biosci 14:351â??365
- Bianchi E and Pessi A (2002). Inhibiting viral proteases: Challenges and opportunities. Pept. Sci; 66:101-114.
- Cao B, Y. Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y and Wei M, Zhang D and Wang C et al. (2020). A Trial of lopinavirâ??ritonavir in adults hospitalized with severe covid-19. N Engl J Med.
- Cohen M, Davydov O and Fluhr R (2019). Plant serpin protease inhibitors specificity and duality of function. Journal of Experimental Botany doi:10.1093/jxb/ery460.
- Cotabarren J, Lufrano D, Parisi MG and Obreg̫̉on WD (2019). Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Science.
- Clemente M, Corigliano MG, Pariani SA, SÃÆánchez-LÃÆópez EF, Sander VA and Ramos-Duarte VA et al. (2019). Plant serine protease inhibitors: biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci; 20:1345.
- Craik DJ (2009). Circling the enemy: cyclic proteins in plant defence. Trends Plant Sci; 14:328-335.
- Craik DJ, Fairlie DP, Liras S, Price D. (2013). The future of peptide-based drugs. Chem Biol Drug Des; 81:136â??147.
- Devaux CA, Rolain JM, Colson P and Raoult D (2020). New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, International Journal of Antimicrobial Agents.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP and Chaicumpa W et al. (2020). COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics, Human Vaccines & Immunotherapeutics.
- Domagala JM, Hagen SE, Lunney E, Arbor A, Tait BD (1996). Coumarin derivatives as protease nhibitors and antiviral agents US Patent: 5,510,375.
- Elliott AG, Delay C, Liu H, Phua Z, Rosengren KJ, Benfield AH, Panero JL, Colgrave ML, Jayasena AS, Dunse KM, Anderson MA, Schilling EE, Barrientos DO, Craik DJ and Mylne JS (2014). Evolutionary origins of a bioactive peptide buried within preproalbumin. Plant Cell; 26: 981â??995.
- Fan SG and Wu GJ (2005). Characteristics of plant proteinase inhibitors and their applications in combating phytophagous insects, Bot Bull Acad Sin; 46: 273â??292.
- Fan Y, Yang W, Yan Q, Chen C and Li J (2020). Genome-wide identification and expression analysis of the protease inhibitor gene families in tomato. Genes; 11:1
- Fang EF, Wong JH, Bah CSF, Lin P, Tsao SW and Ng TB et al. (2010). Bauhinia variegata var. variegata trypsin inhibitor: From isolation to potential medicinal applications. Biochem Biophys Res Commun; 396: 806â??811.
- Fang EF, Wong JH and Ng TB (2010b). Thermostable Kunitz trypsin inhibitor with cytokine inducing, antitumor and HIV-1 reverse transcriptase inhibitory activities from Korean large black soybeans. J Biosci Bioeng; 109: 211â??217.
- Fischer A, Sellner M, Neranjan S, Lill MA and Smiesko M (2020). Inhibitors for novel coronavirus protease identifed by virtual screening of 687 million compounds. ChemRxiv. Preprint. https://doi.org/10.26434/chemrxiv.11923239.v1
- Fischmann TO and Weber PC (2002). Peptidic inhibitors of the hepatitis C virus serine protease within non- structural protein 3. Curr Pharm Des 8:2533-2540.
- Franco OL, Dos Santos RC and Batista JAN (2003). Effects of black-eyed pea trypsin/chymotrypsin inhibitor on proteolytic activity and on development of Anthonomus grandis. Phytochemistry 63: 343â??349.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY and Yan Y (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak â?? an update on the status. Military Medical Research; 7:11.
- Haq SK, Atif SM and Khan RH (2004). Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Arch Biochem Biophys 431: 145â??159.
- Harsulkar AM, Giri AP and Patankar AG, Gupta VS, Sainani MN, Ranjekar PK and Deshpande VV (1999). Successive use of non-host plant protease inhibitors required for effective inhibition of Helicoverpa armigera gut proteases and larval growth. Plant Physiol 121:497â??506.3RRRR
- Headey SJ, MacAskill UK, Wright MA, Claridge JK, Edwards PJB, Farley PC, Christeller JT, Laing WA, and Pasca SM (2010). Solution structure of the squash aspartic acid proteinase inhibitor (SQAPI) and mutational analysis of pepsin inhibition. J Biol Chem; 285:27019â??27025.
- Hejgaard J and Hauge S (2002). Serpins of oat (Avena sativa) grain with distinct reactive centres and inhibitory specificity. Physiol Plant 116:155â??163.
- Hellinger R and Gruber CW (2019). Peptide-based protease inhibitors from plants. Drug Discov Today; 24: 1877â??1889.
- Jain PK and Joshi H (2012). Coumarin: Chemical and Pharmacological Profile. Journal of Applied Pharmaceutical Science; 2: 236-240.
- Jamal F, Pandey PK, Singh D, Khan MK (2013). Serine protease inhibitors in plants: natureâ??s arsenal crafted for insect predators. Phytochem Rev; 12:1â??34.
- Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahm KS and Park Y (2009). Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 2009, 10, 2860-2872.
- Lam SK and Ng TB (2010) A dimeric high-molecular-weight chymotrypsin inhibitor with antitumor and HIV-1 reverse transcriptase inhibitory activities from seeds of Acacia confusa. Phytomed Int J Phytother Phytopharmacol; 17:621â??625
- Laskowski M Jr. and Kato I (1980). Protein inhibitors of proteinases. Annu Rev Biochem; 49: 593-626.
- Lin P and Ng TB (2008). A stable trypsin inhibitor from Chinese dull black soybeans with potentially exploitable activities. Process Biochem; 43: 992â??998.
- Mahoney WC, Hermodson MA, Jones B, Powers DD, Corfman RS, Reeck GR (1984) Amino acid sequence and secondary structural analysis of the corn inhibitor of trypsin and activated Hageman Factor. J Biol Chem 259: 8412â??8416.
- Meng T, Cao H, Zhang H, Kang Z, Xu D, Gong H, Wang J, Li Z, Cui X, Xu H, Wei H, Pan X, Zhu R, Xiao J, Zhou W, Cheng L and Liu J (2020). The transmembrane serine protease inhibitors are potential antiviral drugs for 2019-nCoV targeting the insertion sequence-induced viral infectivity. bioRxiv preprint doi: https://doi.org/10.1101/2020.02.08.926006.
- Mishra UN, Reddy MV and Prasad DT (2020). Plant serine protease inhibitor (SPI): A potent player with bactericidal, fungicidal, nematicidal and antiviral properties. International Journal of Chemical Studies; 8: 2985-2993.
- Moulin MM, Rodrigues R, Ribeiro SFF, Goncalves LSA, Bento CS and Sudre CP (2014). Trypsin inhibitors from Capsicum baccatum var. pendulum leaves involved in Pepper yellow mosaic virus resistance. Genet Mol Res; 13: 9229-9243.
- Nagata K, Norio K, Keiko A, Soichi A and Masaru T (2000). Three-dimensional solution structure of oryzacystatin-I, a cysteine proteinase inhibitor of the rice, Oryza sativa L. japonica. Biochemistry; 39:14753â??14760.
- Oliveira AS, Migliolo L and Aquino RO (2007) Purification and characterization of a trypsinâ??papain inhibitor from Pithecelobium dumosum seeds and itâ??s in vitro effects towards digestive enzymes from insect pests. Plant Physiol Biochem; 45: 858â??865.