Research Article - (2016) Volume 2, Issue 3
Atsushi Fujiwara*, Miki Shimosawa, Masahiko Iino Mikio Sasaki and Shin-ichi Sato
Ina Research Inc. 2148-188 Nishiminowa, Inashi, Nagano 399-4501, Japan
*Corresponding Author:
Atsushi Fujiwara
Ina Research Inc. 2148-188 Nishiminowa
Ina-shi, Nagano 399-4501, Japan
Tel: +81-265-73-8611
Fax: +81-265-73-8612
E-mail: fujiwara@ina-research.co.jp
Received date: August 15, 2016 Accepted date: August 26, 2016; Published date: September 02, 2016
Citation: Fujiwara A, Shimosawa M, Lino M, et al. Physical Dependence and Plasma Concentrations of Morphine in Rats.J Drug Abuse. 2016, 2:3 doi: 10.21767/2471-853X.100032
It is very important to set an appropriate dose level and frequency with a sufficient duration of administration to allow for assessment of the physical dependence liability of drugs. The purpose of this study was to investigate the relationship between the physical dependence liability and blood concentrations of morphine following an oral administration. Morphine was administered by oral gavage once daily for 28 days at dose levels of 10 and 30 mg/kg/day, and then withdrawal signs were observed for 7 days. During the withdrawal period, apparent withdrawal signs such as hyper reactivity to handling, loose stools, piloerection and transient, statistically significant decreases in the percent change of body weight gain and food consumption were noted at 30 mg/kg/day. At 10 mg/kg/day, hyperreactivity to handling and piloerection were observed, but transient decreases in the percent change of body weight gain and food consumption were not noted. The mean plasma concentrations of morphine at 24 h post-dosing at 10 and 30 mg/ kg/day were 3.4 and 13.6 ng/mL on Day 1 of dosing and 3.91 and 20.3 ng/mL on Day 28 of dosing, respectively. The plasma concentrations of morphine at 30 mg/kg/day were above the lower limit of the effective blood concentrations in humans. Thus, it was suggested that dose levels that can maintain effective blood concentrations or higher in humans should be selected for evaluation of physical dependence liability.
Introduction
Abuse liability assessment is necessary for developmental drugs that could potentially affect the central nervous system, and an experiment inducing physical dependence is one such assessment [1-4]. Morphine, well known for its potential to cause physical dependence [5], is a typical representative of dependence producing drugs and various methods have been established in rats to develop physical dependency. For example, in an invasive infusion method, rats implanted with intravenous catheters were treated with morphine at 4 mg/kg/h by continuous infusion or 2-8 mg/kg/infusion by intermittent infusion at intervals of 1 hour for 24-72 h [6, 7] and rats implanted subcutaneous with osmotic mini pumps were treated with morphine at 0.7 mg/kg/h for 72 h [8]. Via the subcutaneous route, rats were injected with morphine at 15-55 mg/kg in a dose-increasing manner once daily for 5 days [9] and at 10 mg/kg twice daily for 5 days [10] or 14 days [11]. Via the intraperitoneal rote, rats were injected with morphine at 1-32 mg/kg in a dose-increasing manner once daily for 6 days [12], at 3 mg/kg once daily for 28 days [12] and at 1-80 mg/kg in a dose-increasing manner twice daily for 6 days [13]. In addition to the above, there are also 3 different methods via the oral route. In a drug-admixed food method, rats were chronically administered morphine-admixed food at 0.5-2 mg/g of food for 7 days [14, 15]. In self-administration via the oral route, rats were self-administered morphine containing tap water or 5% sucrose at 0.1-0.4 mg/mL in a dose-increasing manner for 3 weeks [16]. Via oral gavage, rats received morphine at 50-150 mg/kg/day twice daily in a dose-increasing manner for 40 days [17].
Furthermore, withdrawal signs are induced following spontaneous withdrawal [10, 14] or with the use of antagonistic agents such as levallorphan [15] and naloxone [6-9, 11-13, 16, 17].
When investigating physical dependence, it is recommended to use the clinical route of administration [2]. Various clinical routes of administration are used for morphine, including oral, subcutaneous, intravenous, peridural and intrathecal, but the major clinical route used for developmental drugs is the oral route. Therefore, the potential of morphine to cause physical dependence when administered orally was evaluated in this study. Though regimes of multiple administrations/day have been widely used in research to date, single daily administration was employed in this study to save resources. Spontaneous withdrawal was selected in order to create conditions as close as possible to those clinically observed. Furthermore, since the maintenance of effects on the central nervous system is crucial in order to develop physical dependence, plasma morphine concentrations were determined.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River Laboratories Japan, Inc., Japan) weighing 200 to 221 g were used. These rats were individually housed in stainless steel wire mesh cages (29W × 22D × 21H cm) and had free access to food (CRF-1: Oriental Yeast Co., Ltd., Japan) and water. The room temperature and humidity were set at 23 ± 2°C and 55 ± 15%, respectively. The room was illuminated from 7:00 to 19:00. All experimental procedures were approved and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) of Ina Research Inc., Japan, which is fully accredited by AAALAC International.
Procedures
Induction of physical dependence and observation of withdrawal signs of morphine
Morphine was administered by oral gavage to 10 rats/group once daily for 28 days at dose levels of 10 and 30 mg/kg/day followed by a 7 day withdrawal period. Ten negative control rats received water for injection at 10 mL/kg/day in a same manner. Gross behavioral observations and measurements of body weights and food consumption were conducted at intervals of 3 to 4 days during the dosing period and once daily during the withdrawal period. Gross behavioral observations were conducted according to the methods and criteria of our laboratory. Observation parameters included continual movement, posture (prone position, lateral position), piloerection, hyper- or hypo-reactivity to handling, slowed motion, ataxia and muscle relaxation, loose stools and diarrhea (muddy or watery stools) during the dosing period and piloerection, head shaking, wet-dog shaking, jumping, chewing, teeth chattering, licking, salivation, hyperreactivity to handling, tremors and convulsions, loose stools and diarrhea (muddy or watery stools) during the withdrawal period.
Determination of plasma morphine concentrations
Plasma samples were collected at 0.5, 1, 2 and 24 h after dosing with morphine from 3 rats each in the 10 and 30 mg/kg/day groups on Days 1 and 28 of dosing. The plasma morphine concentrations were determined by LC-MS/MS (API4000, AB SCIEX Ltd, Japan).
Drugs
Morphine (Morphine hydrochloride hydrate, Takeda Pharmaceutical Industry, Japan) was dissolved in water for injection (Otsuka Normal Saline, Otsuka Pharmaceutical Factory Inc., Japan) and kept in a light-resistant container under refrigeration.
Data analyses
The percent changes of body weight gain during the withdrawal period were calculated as follows: [(Body weight on each day of withdrawal − Body weight on Day 28 of dosing)/Body weight on Day 28 of dosing] × 100.
The body weights, percent changes of body weight gain and food consumption were analyzed for homogeneity of variance using Bartlett’s test (5% level of significance) between the negative control and each treated group. When the variance was homogeneous between the groups, mean group values were compared using Dunnett’s test. When the variance was heterogeneous based on Bartlett’s test, Steel’s test was conducted.
Results
Dosing period
No gross behavioral changes were observed in any groups in gross behavioral observations. Statistically significant decreases in body weights and food consumption were noted in both treated groups from Day 4 or 8 as compared to the negative control group. In the 10 mg/kg/day group, food consumption recovered from Day 15 (Figure 1).
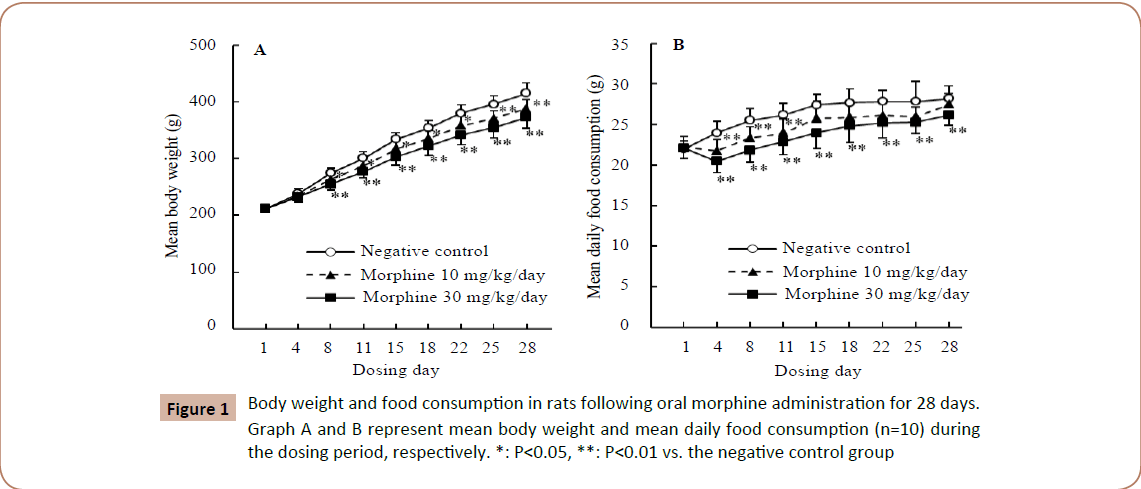
Figure 1: Body weight and food consumption in rats following oral morphine administration for 28 days. Graph A and B represent mean body weight and mean daily food consumption (n=10) during the dosing period, respectively. *: P< 0.05, **: P< 0.01 vs. the negative control group
Withdrawal period
In the gross behavioral observations (Figure 2A), gross behavioral changes such as hyperreactivity to handling and piloerection were observed dose-dependently. Diarrhea and loose stools were also observed in the 30 mg/kg/day group, but not in the 10 mg/kg/day group. The peak of these signs was noted on Day 3 of withdrawal.
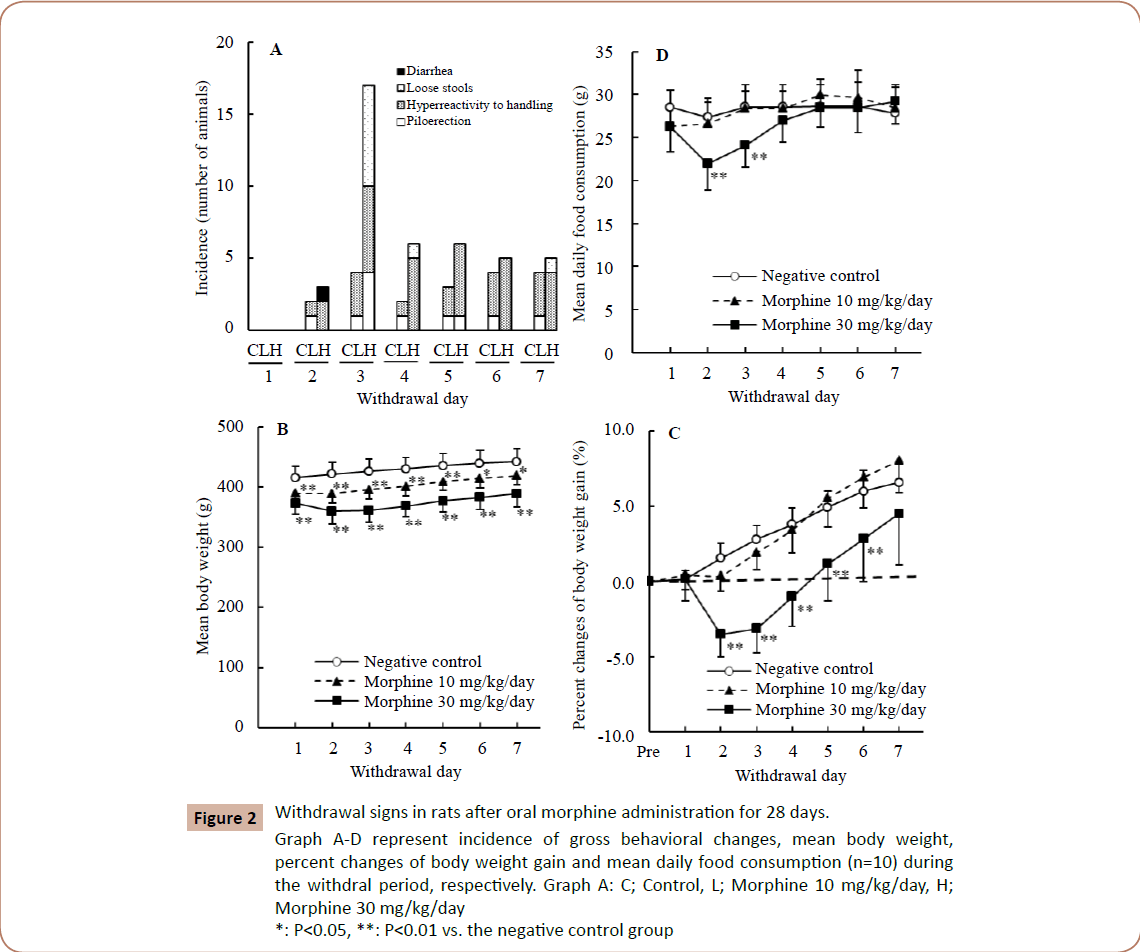
Figure 2: Withdrawal signs in rats after oral morphine administration for 28 days. Graph A-D represent incidence of gross behavioral changes, mean body weight, percent changes of body weight gain and mean daily food consumption (n=10) during the withdral period, respectively. Graph A: C; Control, L; Morphine 10 mg/kg/day, H; Morphine 30 mg/kg/day *: P < 0.05, **: P < 0.01 vs. the negative control group
In the body weights (Figure 2B), the statistically significant decreases noted in the treated groups during the dosing period persisted in the withdrawal period, with transient decreases noted in the 30 mg/kg/ day group on Days 2 and 3 of withdrawal.
Withdrawal period
As for percent changes of body weight gain (Figure 2C), statistically significant decreases on Days 2-6 of withdrawal and transient decreases on Days 2 and 3 of withdrawal were noted in the 30 mg/kg/day group, but no such changes were noted in the 10 mg/ kg/day group. The maximum decrease in body weight gain was 3.5% on Day 2 of withdrawal.
In the daily food consumption (Figure 2D), statistically significant transient decreases were noted in the 30 mg/kg/day group on Days 2 and 3 of withdrawal, but no such changes were noted in the 10 mg/kg/day group. The maximum decrease in food consumption was 13% on Day 2 of withdrawal.
Determination of plasma morphine concentrations
The mean plasma concentrations of morphine increased almost dose-proportionally (Table 1). Each parameter showed higher exposure levels on Day 28 than on Day 1. According to the C24h values, the plasma concentrations were maintained at more than 3.4-3.9 and 13.6-20.3 ng/mL in the 10 and 30 mg/kg/day groups, respectively.
| Morphine Group |
|
||||
| n | Day | Cmax(ng/ml) | Tmax (h) | C24h (ng/ml) | |
| 10 mg/kg/day | 3 | 1 | 58.9 | 1 | 3.4 |
| 28 | 112 | 1 | 3.9 | ||
| 30 mg/kg/day | 3 | 1 | 151 | 1 | 13.6 |
| 28 | 466 | 1 | 20.3 | ||
Table 1: Plasma concentration of morphine during the dosing period.
Discussion
In this study, the relationship between the development of physical dependence and plasma concentration of morphine was investigated following a single daily oral administration.
The withdrawal signs of morphine are well demonstrated by Yanaura et al. [14]. In this report, withdrawal signs were observed in male Sprague-Dawley rats that received morphine admixed-food for 1 week at 0.5 and 1 mg/g of food for the lower dose group, and 1 and 2 mg/g of food for the higher dose group followed by the provision of normal food to observe the withdrawal signs. During the spontaneous withdrawal period, diarrhea, loose stools, decreases in body weights (11.6%) and food consumption (67.6-70.5%) in the lower dose group, and ‘wet dog’ shakes and hyper reactivity to handling, decreases in body weights (12.8%) and food consumption (91.7%) in the higher dose group were noted. In this study, similar withdrawal signs such as hyper reactivity to handling, loose stools, diarrhea, decreases in body weights and food consumption were noted during the withdrawal period following the administration of morphine at 30 mg/kg/day, suggesting morphine-induced physical dependence under these experimental conditions.
However, the degree of the decreases in body weights and food consumption were very slight in this study. The administration regimen, such as the dose levels, dosing intervals and duration, is very important to induce physical dependence [1] and the regimen affects the degree of physical dependence and withdrawal signs. When morphine was subcutaneously administered at 12 mg/kg/ day to rhesus monkeys, the degree of withdrawal signs at 3 mg/ kg × 4 times daily and 6 mg/kg × twice daily was severer than those at 12 mg/kg × once daily. In addition, development of physical dependence using morphine at 3 mg/kg × 4 times daily and 6 mg/kg × twice daily was faster than at 12 mg/kg × once daily [18]. These results indicate that maintenance of the effects of morphine induces stronger physical dependence. The plasma and serum concentrations of morphine in the development of physical dependence have been previously reported in rats. In subcutaneous implantation of morphine pellets, the degree of morphine physical dependence was directly related to the AUC and Cmax in serum concentrations [19] and maintained plasma concentrations of morphine [20]. Decreases in the body weights of 5 and 10% were noted when the plasma concentrations of morphine were 100 and 200 ng/mL, respectively [20]. In the drug-admixed food method, the peak serum concentrations of morphine in the 1 and 2 g food groups were approximately 400 and 800 ng/mL. Furthermore, a decrease in the body weights of 10% and a decrease in food consumption to 60% were observed as withdrawal signs in both these groups [21]. Although the plasma and serum concentrations of morphine in the drug-admixed food method changed depending on the time of food intake, morphine physical dependence was developed when plasma morphine levels were maintained at more than 100 ng/mL [22]. Thus, it is considered that maintenance at or above a particular dose level is necessary in the development of morphine physical dependence, rather than the maintenance of constant blood concentration levels. Since the current study utilized single daily oral dosing, fluctuations in plasma concentrations were larger than in the drug-admixed food method and morphine concentrations were maintained at low levels (13.6-20.3 ng/mL or above). It is thought that the decreases in body weights and food consumption were slight as a result of these low concentrations.
In tail-flick and hot-plate tests in rats, antinociceptive effects were noted at a morphine serum concentration of more than 100 ng/mL [23, 24]. This concentration is the same as that mentioned above as inducing physical dependence when maintained in the drug-admixed food method. Therefore, by selecting a dosing regimen based on plasma concentrations at which analgesia is attained, it is possible to induce stronger withdrawal signs. On the other hand, the lower limit of plasma concentrations for effective pain relief in humans is between 15 and 65 ng/mL [25], comparable to the plasma concentration of morphine maintained in the current study of between 13.6 and 20.3 ng/mL. This indicates that the development of physical dependence based on the concentrations effective in humans may only produce weak withdrawal signs. Therefore, when selecting dose levels based on effective plasma concentrations in humans, it is necessary to also select higher dose levels for investigation.
In summary, morphine-induced physical dependence following an oral administration at 30 mg/kg/day once daily for 28 days. In this regimen, the plasma concentrations of morphine were maintained above the lower limit of the effective blood concentration in humans.
Acknowledgement
The authors thank Ms. Misato Okada, Ms. Kazue Aruga and Ms. Penelope Naruta for providing technical assistance.
Conflicts of Interest
None.