Review Article - (2018) Volume 4, Issue 3
Alexander Berezin*
Department of Internal Medicine, State Medical University for Zaporozhye, Ukraine
*Corresponding Author:
Alexander Berezin
Department of Internal Medicine
State Medical University for Zaporozhye
26, Mayakovsky Av., Zaporozhye, Postcode 69035, Ukraine
Tel: +38 061 2894585
Fax: +38 0612894585
Email: dr_berezin@mail.ru
Received Date: September 18, 2018; Accepted Date: October 06, 2018; Published Date:October 15, 2018
Citation: Berezin A (2018) Pattern of Micro Vesicles in Heart Failure: Novel Biomarker of Endothelial Dysfunction and Vascular Reparation. Biomark J. 4:16. doi: 10.21767/2472-1646.100054
Heart failure (HF) is a global public health concern that continues to have a sufficient impact on morbidity, mortality and disability in developing ad developed countries. Contemporary diagnostic and prediction strategy bases on biomarker use, which helps improving individual prognostication and early verification of the disease. However, widely implementing biomarkers such as natriuretic peptides, and several new biomarkers (glectin-3, soluble ST2 and others) were not demonstrated strong ability to improve discriminative capacity in various HF phenotypes. In this context micro vesicle (MVs), which play a core role in the endogenous reparation, coagulation, inflammation, immunity and metabolic memory phenomenon, could be prospective biomarkers in HF with predictive ability. However, there is not yet strong evidence those immune phenotypes of MVs are important for prediction and development of several phenotypes of HF. The aim of the review: to summarize knowledge regarding the role of various MVs in diagnosis and prognosis of HF.
Keywords
Heart failure; HFpEF; HFmrEF; HFrEF; Micro vesicles; Diagnosis; Prediction
Abbreviations
BMI: Body Mass Index; BNP: Brain Natriuretic Peptide; CHF: Chronic Heart Failure; CV: Cardiovascular; EVs: Extracellular Vesicles; HF: Heart Failure; HFmrEF: Chronic HF with Mid-Range Ejection Fraction; HFpEF: Chronic HF with Preserved Ejection Fraction; HFrEF: Chronic HF with Reduced Ejection Fraction; HSP: Heart Shock Protein; GFR: Glomerular Filtration Rate; hs-CRP: High Sensitive C-Reactive Protein; HDL-C: High-Density Lipoprotein Cholesterol; ICAM: Intracellular Adhesion Molecule; LDL-C: Low- Density Lipoprotein Cholesterol; LVEF: Left Ventricular Ejection Fraction; MVs: Micro Vesicles; MI: Myocardial Infarction; STEMI ST: Segment Elevation Myocardial Infarction; PGF: Placental Growth Factor; PLGA: Poly Lactic-co-Glycolic Acid; VCAM: Vascular Cell Adhesion Molecule; VEGF: Vascular Endothelial Growth Factor
Introduction
Although last decades shown the prevalence of heart failure (HF) tend to be decreased in developed countries, morbidity and mortality of this disease remains unacceptably high [1,2]. Additionally, due to a higher incidence of recurrent admission to the hospital global expenditures for HF care are discussed as extremal and exaggerate [2,3]. Current European clinical guideline for HF determines three phenotypes of HF, such as HF with reduced left ventricular ejection fraction (HFrEF), HF with preserved (HFpEF) left ventricular ejection fraction and HF with mid-ranged (HFmrEF) left ventricular ejection fraction, which exhibited increased risk of death and HF-related clinical outcomes including re-hospitalization compared to individuals who did not have HF [1,4]. Keeping in mind HFrEF patients had the worst longterm clinical prognosis, but HFpEF could show better survival rate. In contrast to the patients with HFrEF and HFpEF individuals with HFmrEF generally had intermediate clinical characteristics, but the risk of death was similar HFrEF [5,6]. Interestingly, the survival rate may relate to age, sex, ethnicity, socioeconomic status and co-morbidities in any patients with HF regardless of phenotype of the disease [5,6]. However, clinical outcome in all HF categories and require to be categorized as poor.
The clinical correlates with prognosis sufficiently distinguished each other in patients with various phenotypes of HF and associated with scattered risk marker profile that is explained suggesting differences in underlying pathophysiological mechanisms. For instance, patients with HFrEF were more likely to have male gender, current smoker status, coronary disease, previous myocardial infarction, but female gender, atrial fibrillation, obesity, elevated urinary albumin excretion and cystatin C levels were frequently conferred HFpEF or HFmrEF [7]. However, any phenotypes of HF were determined with increased N-terminal pro-B-type natriuretic peptide (NT-proBNP) [8]. Interestingly, the clinical outcomes in patients with different HF phenotypes have been occurred quite similar and that is important challenge for current medical service [9-11].
Despite improving the HF care remains a priority for health professionals worldwide and novel technologies (cardiac resynchronization therapy, implantable defibrillator/ cardioverters, regenerative cell therapy, personified translational care) are more and more implemented into routine clinical practice, the HF bedevils a devastating impact on patients and prognosis of the disease remains to be poor [10,11].
Although several studies have reported the utilization of numerous biological markers in HF for improving diagnosis, prediction, and therapy, but natriuretic peptides, galectin-3, soluble ST2, and high sensitive cardiac specific troponins were validated only in US and natriuretic peptides are recommended in EU [4,12]. Indeed, both 2012 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure and 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Guideline for the Management of Heart Failure are positively accepted by many clinicians in different countries as reliable and simple tool for diagnosis and prognosis of suspecting HFrEF, HFmrEF and HFpEF [13,14]. At the same time, individualized risk stratification and personalized medical care remain challenging for many patients with established HF, because current diagnostic and medical strategy based on universal clinical criteria and biomarker approaches [15]. In fact, NPs are universal diagnostic biomarkers, while other biomarkers that were disputed in the clinical guidelines mentioned above including ST-2, galectin-3, growth differentiation factor-15 mid-regional prohormone are emerging as potentially useful prognostic markers in HF. However, there is not strong evidence that all these biomarkers are best fitted for monitoring of response in medical care in HF. Indeed, most of the research on biomarkers in HF has appropriately focused on their use as diagnosis and predictive tools, another emerging role of biomarkers predominantly NPs and galectin-3 is as part of the design of several clinical trials, which are focused on biomarkers as surrogates in HF [16]. Current findings reported that NPs and galectin-3 are biomarkers the concentrations of which have demonstrated a potency to increase depending on advance of HF and to decrease in resulting of euvolemic state achievement. However, high biological variability, depending on age, metabolic comorbidities and kidney function, as well as lowered ability to predict different phenotypes of HF is considered as a serious limitation for serial measures of traditional biomarkers [17].
To note, wide spectrum of novel biological markers reflecting different stages of nature evolution of HF could stratify patients at risk and translating medical care based on individual-derived particularities of the disease including phenotype of HF, etiology, co-morbidities, and complications [18,19]. Additionally, micro vesicles (MVs), which are known as biomarker of vascular injury and endothelial dysfunction appear to be best fitted to personified biomarker stratification at risk of HF developing and HF-related events [20-22]. The aim of the review is to summarize new knowledge for the role of various MPs in diagnosis and prognosis of HF.
Definition, classification, structure and regulation of micro vesicles
Table 1 reports the basic characteristic of sub-population of extracellular vesicles (EVs) with diameter average from 100 to 1000 nm originated from circulating cells. EVs are determined phospholipid-based endogenously produced particles (30- 1000 nm in diameter), which contain cell-specific proteins, glycoproteins, lipids, nucleic acids and active molecules. Ability to secret EVs is proven for numerous cells including circulating blood cells cardiomyocites, mature endothelial cells, immune cells (including T- and B-lymphocytes, dendritic cells), astrocytes, and tumor cells.
| Population of vesicles | Diameter, nm | Origin | Main contained components | Best characterized cellular sources | Markers |
|---|---|---|---|---|---|
| EV | 30-1000 nm | cell membranes | regulatory proteins (i.e., heat-shock proteins, tetraspanin), lipids, active molecules, nucleic acids (mRNA, miRNA), cytokines, growth factors, hormones, procoagulant phosphatidylserine, likely complement | All cell types | Annexin V binding, tissue factor and cell-specific markers |
| Platelets, RBC and endothelial cells | |||||
| MV | 50–1000 nm | plasma membranes | Platelets, RBC and endothelial cells | ||
| Small-size MPs | <50 nm | plasma membranes | Endothelial cells | CD133+, CD63- | |
| Exosomes | 30–100 nm | endosomal membranes | Immune cells and tumors | CD63, CD61, CD63, CD81, CD9, LAMP1 and TSG101 | |
| Ectosomes | 100–350 nm | plasma membranes | Platelets, RBC, activated neutrophils, and endothelial cells | TyA, C1q | |
| Late endosomes | 50–1000 nm | endosomal membranes | close-packed lumenal vesicles | Immune cells and tumors | Annexin V binding, DNA content |
| Apoptotic bodies | 0.5-3.0 µm | plasma membranes | Pro-apoptotic molecules, oncogenic receptors | Cell lines |
Abbreviations: EVs, extracellular vesicles; MPs, micro particles; MV, micro vesicles; RBC, red blood cells.
Table 1: Classification and key features of extracellular vesicles.
There are numerous classifications of EVs depending on origin, sizes, immune phenotypes, and triggers that induce EV realize. For instance, subsets that originated from endosomal membrane are named exosomes, particles that are released on the exocytosis are called late endosomes, EVs released from the plasma membrane are identified as ectosomes, and finally actively secreting EVs as a component of cell membrane are named micro vesicles (MVs). Exosomes have average size from 30 to 100 nm in diameter, the MVs are usually determined as particles with 50 to 1000 nm in diameter, ectosomes have diameter in average from 100 to 350 nm, small-size MVs and apoptotic bodies have diameter 50 nm and less [23-25].
Ninety percent and more of MPs in healthy volunteers are originated from platelets, 10% of MV population is reported as granulocytes-derived particles and about 5% of MCs are reported as endothelial cell-derived MVs, red blood cells (RBCs) and monocytes [26]. Since all types of particles contain surface proteins derived from their cell of origin (including antigen-presenting cells), while there are additional biomarkers confirming origin of the MVs (Figure 1). Table 2 reports the most common features of different MV subsets. It has suggested that the difference between number and immune phenotypes of several subsets of MVs could use as promising biomarker with predictive value of CV diseases including HF.
| Subsets of MVs | Markers | The most common methods of detection | |
|---|---|---|---|
| Derived from resting or activated cells | |||
| Granulocytes | CD24+CD11c− CD66b/CD66acde | Flow cytometry western blotting, mass spectrometry, electron microscopic technique, SPRi microscopy | |
| Monocytes | CD14 | ||
| Microphages | CD11b+ CD64+/− Ly6Clo | ||
| Endothelial cells | CD144, CD62E | ||
| T cells | CD4 or CD8 | ||
| B cells | CD20 | ||
| Dendritic cells | CD1a, CD14, CD141, CD80, CD85, CD86 | ||
| ICAM(+) cells | CD54 | ||
| VCAM(+)cells | CD106 | ||
| Platelets | CD41 and/or CD61 | ||
| RBCs | CD235a, CD44, CD47, CD55, CFSE, annexin V and anti-glycophorin A | ||
| Derived from activated or tumor cells | Annexin V binding, CD63, CD81, CD9, LAMP1 and TSG101 | Flow cytometry, capture based assays | |
| Derived from apoptotic cells | Annexin V, DNA content, histones | Flow cytometry | |
Abbreviations: ICAM, intracellular adhesion molecule; VCAM, vascular cell adhesion molecule; SPRi microscopy, nano-particles- surface plasmon resonance - based imaging microscopy; CFSE, carboxyfluorescein diacetate succinimidyl ester.
Table 2: The most common features of MV subsets.
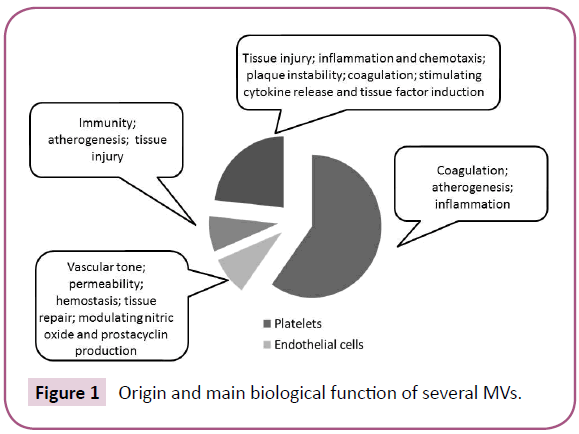
Figure 1: Origin and main biological function of several MVs.
Biological role and function of MPs
MVs have great potentiality in material applications [27], but initially they were found as cell debris and they biological function was not recognized completely. Developing analytic technologies attenuate recognition, determination, and measures of several subsets of MVs [28].
It has been postulated that MPs are a cargo for various molecules including proteins, RNAs, micro-RNAs, DNA fragments, lipids, active molecules, hormones, growth factors. MPs play a central role in cell-to-cell cooperation via transfer these substances from mother cells to recipient cells. Within last decade MPs were found as independent mediator of cell function regardless of cargo. For instance, MC producing with activated cells or apoptotic cells may effect mutually opposite impact of target cells attenuating differentiation and growth or leading to weak function and cell injury [29-31]. Gaining evidence of a pivotal role of MVs originated from different cells (RBCs, mononuclears, endothelial cells, platelets, immune cells) in nature evolution of various diseases including CV diseases, cancer, sepsis, pre- and eclampsia, autoimmune and metabolic states [32-35].
Mononuclear cell-derived MVs are involved in several processes including inflammation, blood coagulation, and thrombosis [36,37]. Mononuclear cells are able to release MVs with proinflammatory capacities as resulting in activation p38 mitogenactivated protein kinase signaling due to effect of several cytokines, bacterial antigens, P-selectin, histamine, catecholamines, angiotensin-II, and cigarette smoking [38-42]. Additionally there is spontaneous secretion of MVs by mononuclear cell without obvious cause in physiological state [42-44].
The most common mechanisms by which MVs may exert their biological functions on target cells are reported (Figure 2).
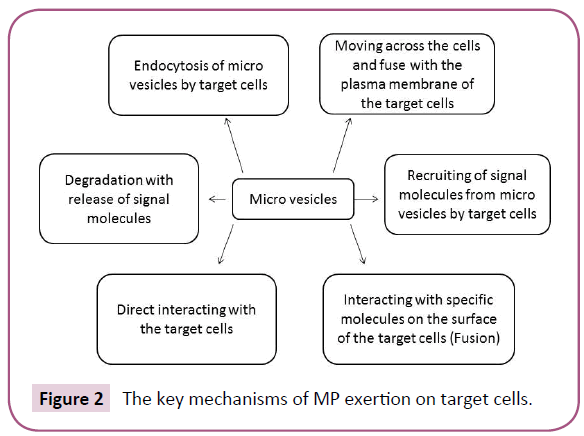
Figure 2: The key mechanisms of MP exertion on target cells.
MVs originated from mononuclears and mature endothelial cells may incorporate in several places of endothelium and damages its integrity directly or via cooperation with oxidized lipids [45- 47]. It is interesting that older individuals demonstrated higher level of pro-coagulant mononuclear cell-derived MVs compared with young people in physiological state and in diseases [48]. The most common triggers for mononuclear cell-derived and endothelial cell-derived MVs were laminar shear stress on endothelium, antigen stimulation, coagulation/thrombosis, endotoxins, activated/apoptotic cells, ischemia/hypoxia and malignancy [49-53].
Finally, mononuclear cell-derived and endothelial cell-derived MVs play an important role in cell-to-cell cooperation, vascular reparation, neovascularization, angiogenesis, oxidative stress, inflammation, and coagulation [53-55].
MVs originated from RBCs act as predominantly trigger of coagulation [56] that has been found in several states including stroke, acute coronary syndrome, myocardial infarction, acute HF, hemolysis, sickle cell disease, thalassemia, chronic kidney disease, trauma, and sepsis [57-62]. Therefore, RBC-derived MPs may be produced ex vivo during cold storage of RBCs because they present factor XI on their surface [63]. All these findings confirm the hypothetical role of RBC-derived MVs in attenuation of coagulation cascade and promoting thrombotic state [64-66]. On the other hand, RBC-derived MVs have fibrinolytic activity predominantly related to plasminogen content on surfaces of them [67].
Koshiar et al. [68] reported that RBC-derived MP surface contains protein C system, which act as natural anticoagulant in vivo. Nevertheless, cell-free RNAs, which are derived from RBCs-MPs, play a pivotal role in transfusion-related immune modulation [56,69].
Platelet-derived MVs are produced due to activation, stress, or apoptosis of platelets like several types of nucleus cells. Because platelet-derived MVs contain phospholipid-based membranes and express in numerous receptors on their surface, they are found as pro-coagulant and complement triggers. The most common biological role of platelet-derived MV is regulating hemostasis, coagulation, inflammation, and probably promoting tissue regeneration and cell repair [70-73].
Table 3 is accumulated information about established role of different subsets of MVs in several diseases. Additionally, Figure 3 is reported the crucial role of MVs in physiological events occurring in tissues and organs.
| Subsets of MVs | Relation to pathogenic processes | Relation to diseases |
|---|---|---|
| Platelet-derived MVs | Coagulation, inflammatory processes, thrombosis, and malignancy | ACS, myocardial infarction, HF, tumor progression, systemic lupus erythematosus, vasculitis |
| Leukocytes-derived MVs | Endothelial dysfunction, , and vascular inflammation | CV diseases, infections, sepsis, autoimmune disease |
| Endothelial cell-derived MVs | Endothelial dysfunction, angiogenesis, tumor growth, and increased oxidative stress | Hypertension, HF, CV diseases, diabetes, metabolic syndrome, obesity, vasculitis, sepsis, inflammation, thrombosis, pulmonary hypertension, acute kidney injury, chronic kidney disease, antiphospholipid syndrome, preeclampsia |
| RBC-derived MVs | Immunomodulation, coagulation, inflammation | Thrombosis, vasculitis, sepsis, ACS, myocardial infarction, autoimmune disease, chronic kidney disease, antiphospholipid syndrome, preeclampsia, graft rejection |
| Immune cells-derived MPs | Cargo of tumor rejection antigens | Tumor progression |
Abbreviations: MVs, micro vesicles; CV, cardiovascular disease; ACS, acute coronary syndrome; RBC, red blood cells.
Table 3: The critical role of MVs in pathogenesis of several diseases.
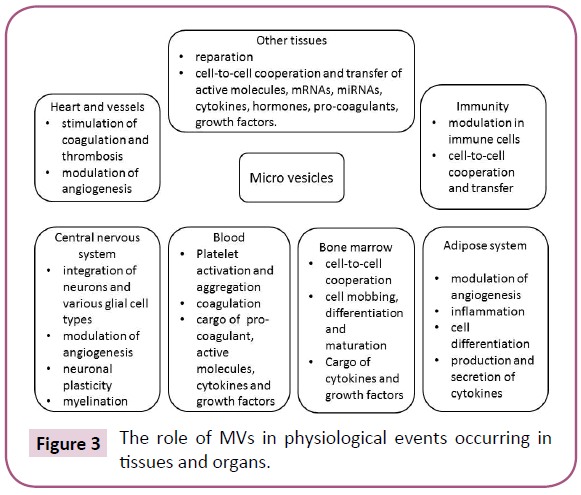
Figure 3: The role of MVs in physiological events occurring in tissues and organs.
Platelet-derived MVs were found a cargo tool of bioactive molecules including growth factors, signaling molecules and fragments of chromatin [74].
Endothelial cells-derived MVs are produced by several triggers such as angiotensin II, lipopolysaccharide substances, and hydrogen peroxide [49]. Because all these factors are central players in the pathogenesis of CV diseases, it is not surprising that endothelial cells-derived MVs are involved in the target organ damage, either exacerbating disease progression or triggering a repair response [75-79]. Noted that endothelial cells-derived MVs (EMVs) originated from apoptotic cells had chromatin substance and may destroy target cell directly, promote coagulation/ thrombosis [75,76]. In contrast, EMVs derived from activated mature endothelial cells do not contain chromatin components and have angiogenic properties contributing to tissue repair [77-80]. In this context, origin of endothelial cells-derived MVs might have the crucial for vascular integrity and supporting endothelial function. All these are recognized as an important mechanisms of developing CV disease and HF for which endothelial dysfunction is key element in pathogenesis of the diseases.
Measure of MVs
There are several controversies in point of view on methodology of assay of morphology, transcriptomics, and proteomics of circulating MVs [81,82]. Indeed, there is sufficient difficulty in separating and isolation of MVs. All these depend on the limits of detection of conventional flow cytometry, and other analytical methods that enable determine of MVs in biological fluids and recognize of their origin [83]. Therefore, the majority of methods of MV detection remain costly and time consuming [84].
The conventional approach for measuring the MVs is based on commonly used flow cytometry, mass spectrometry-based proteomic methods, Western blot analysis, electron and atomic microscopy, and nano-particle tracking analysis (NTA) [85]. All of them demonstrate serious limitations in sensitivity, specificity, probability and accuracy in resolution of MVs, while commercial flow cytometry technique remains gold standard at this way [86,87].
Flow cytometry is a well-standardized and accepted method for cell identification and detection, although the standard tool requires special attention when measuring MVs in diameter less 200 nm [88]. Therefore, there is serious limitation regarding ability to recognized small-size particles like MVs in diameter, i.e. low-density lipoproteins. However, the flow cytometer provides the possibility to measure MPs directly in plasma samples and to analyze MV-subsets [89].
NTA technique is commercially available method that allows visualizing MV and measuring number of them, while detection limit for this method is 50 nm. Western blot analysis, atomic and electron microscopy require high qualified team with great experience in this field and are much expensive. To overcome size limit highly sensitive fluorescent (HSF) microscopy and advanced flow cytometry technique based on BD Horizon Violet Proliferation dye are used now as alternative techniques. However, two and more consequently performing analytic techniques appear to be much more promising in detection of wide-range of MVs [86,87]. Probably nano-particles- surface plasmon resonance-based imaging microscopy (SPRi microscopy) could be simple and affordable method for MV determining in future [88-90]. However, SPR signal between cell edges and substratum sufficiently improves identification of MV edges and segmentation of MV areas [91]. Probably simultaneous utilization of a HSF microscopy and SPRi microscopy could enhance the sensitivity and selectivity of novel biosensor platform besides in detection of small sized MVs [92-96].
Additionally, there are not commercially available techniques that could be promising in MV detection, such as surface-assisted laser desorption/ionization mass spectrometry (SALDI-MS) Raman micro-spectroscopy, micro nuclear magnetic resonance technique, small-angle X-ray scattering, and anomalous smallangle X-ray scattering [97,98]. The main advantage of these approaches is detection of MVs isolated from multiple biological sources without previous sample preparation per protocols. There is integrated system entitled highly sensitive magnetic resonance imaging that allows detecting MV antigens with microfluidic chip and labelling target-specific magnetic nanoparticles [99]. Thus innovative approach may differentiate MVs derived from tumor cells from non-tumor host cells [99].
There are commercial platforms that offer simultaneous, labelfree optical biological sensing of MVs with affordable integrated analytic circuits [100]. Despite these successes, isolation, purification and content analysis of MVs remain to be failed to use in routine laboratory practice [101]. Finally, any single method of MV determination does not appear to be superior a combined methods. Novel models and techniques for identification of MVs based on real-time and label-free biosensors, such as SPR, demonstrate advantages before conventional old methods.
MVs in Cardiovascular Disease
Number of circulating MVs originated from several cells is changed in patients with known CV disease including HFrEF, HFmrEF and HFpEF [102,103]. Recent studies have shown elevated levels of MVs originated from numerous blood cells MVs was found in the patients with established CV disease including HF compared with healthy volunteers [102-107]. However, there are several controversies about the role of MVs originated from various blood cells in HF.
RBC-derived MPs
Previous evidence has addressed to the role of erythrocytederived MVs (RBC-derived MVs) in coagulation, inflammation, and immunity, but later some suggestions about direct involvement of RBC-derived MVs in the pathogenesis of HF begin to appear. Indeed RBC-derived MVs are mobilized from gaining thrombi into the distal blood flow in STEMI and may be detected in peripheral blood [108]. Probably, newly and ongoing thrombosis could determine via measure of pattern of RBCs-derived MVs [108,109].
Sansone et al. [110] reported that there were elevated levels of RBC-derived MVs, WBC-derived MVs endothelial cell-derived MVs and platelet-derived MPs in patients after implantation of left ventricular mechanical support devices. Authors suspected that mechanical supporting can be a causing factor leading to this phenomenon. In contrast, there is evidence that there was no significant difference between number of RBC (CD235a+)- derived MVs in patients with sudden cardiac death due to STEMI and individuals with stable coronary artery disease [111].
However, numerous findings confirm the causality role of elevated RBCs-derived MVs in CV events resulting in blood transfer, hemoglobinopathies, transplant rejection and autoimmune conflict. Unfortunately, causative role of RBCs-derived MVs in CV disease and HF in not established completely.
White blood cells-derived MVs
The white blood cells-derived (WBCs) MPs were found in higher concentrations in the patients with acute and chronic coronary syndromes, HF and metabolic disease including T2DM, abdominal obesity [112-114]. There is evidence that several immune phenotypes like CD14(+) of WBCs-derived MVs were involved in the reparative vascular response to injury in acute coronary syndromes [113]. Morel et al. [114] found that primary PCI treated patients with STEMI had significantly higher levels of leukocytederived CD11a(+) MVs, endothelial cell-derived CD105(+) MVs, and tissue factor-bearing MVs than those who did not treat with PCI [114]. Thus, elevated WBC-derived MVs suggested their pathophysiological role in coronary occlusion in STEMI. Therefore, there is evidence that generation of pro-coagulant mononuclear cells-derived MVs might be under control of leptin, visfatin and other metabolic triggers [115]. Overall, WBC-derived MVs are a marker of cell injury, inflammation and coagulation. Whether these phenotypes of MVs are promising indicator of a risk in HF is not yet clear ane requires to be investigated in large clinical studies.
Platelet-derived MVs
Platelet-derived MVs were found in higher concentrations in numerous diseases including acute coronary syndrome/ myocardial infarction, HF, heparin-induced thrombocytopenia, thrombotic thrombocytopenic purpura, pulmonary thromboembolism, and hemolytic uremic syndrome. At the same time, there was not determined a significant difference between levels of platelet-derived MVs in healthy volunteers and patients with known abdominal obesity, T2DM, antiphospholipid syndrome, infection disease or sepsis [116]. Probably, platelets before secretion of MVs require to be activated with of adhered WBCs or endothelial cells [117].
Although the importance of platelet-derived MVs in HF pathogenesis remains unclear [116-119], there is evidence that platelet-derived MVs attenuate shaping foam cells in atherosclerotic plaque and thereby may contribute plaque rupture and acute coronary syndrome, which are known predictors of HF [120]. However, some fractions of MVs derived from platelets expressed P-selectin and/or CD63 and play a pivotal role in thrombus formation and atherothrombotic events [121,122]. Michelsen et al. [123] reported that the elevated level of platelet-derived MPs in survivors of acute myocardial infarction associated strongly with thrombosis and soluble CD40 ligand determined in peripheral blood. Probably, MVs derived from platelets are a factor, which directly mediates endothelial dysfunction and accelerate atherosclerosis, while plateletderived MVs may cooperate with endothelial progenitor cells in vascular reparation [116,120,122-124]. Indeed, platelet-derived MVs are able to interact with early outgrowth cells and prevent vascular injury with modulating regenerative ability of progenitor cells [125,126]. Baj-Krzyworzeka et al. [127] shown that plateletderived MVs attenuated biological functions of hematopoietic cells that potentiated intercellular cross-talk in hematopoiesis and vascular repair. Ohtsuka et al. [128] reported that MVs originated from platelets improved endothelialization of capillary with involving angiogenic progenitor cells into regeneration places. Thus, platelet-derived MVs are promising biological marker for vascular dysfunction and CV events that may be incorporated into program of risk stratification in patients with known CV diseases and probably HF [129].
Endothelial cells-derived MVs
There is large body of evidence that the number of endothelial cells-derived MVs (EMVs) may be a marker of endothelial dysfunction with predictive value for CV events and diseases. Recent clinical studies have shown that number of CD31+/ annexin V+ EMVs associated with altered endothelial function and clinical outcomes in patients with stable coronary artery disease [130,131]. Additionally, Huang et al. [132] reported that both increased number of circulating CD31+/annexin V+ EMVs and lowered number circulating EPCs are able to predict a CV risk in hypertension. The increased number EMVs in peripheral blood were found in individuals with acute coronary syndrome and it correlated well with a risk of sudden cardiac death [112]. Nevertheless, a number of EMP with immune phenotype CD42- CD31+ inversely associated with microvascular obstruction grading in acute myocardial infarction [133]. Additionally, impaired ratio between number of apoptotic EMVs and EMVs originated from activated mature endothelial cells was elucidated in numerous patients with known HF, established CV disease as well as in patients at high risk of CV disease and events [124,134,135]. This finding has now recognized a marker of vascular dysfunction due to several reasons including inflammation, thrombosis, atherosclerosis, and contributed to a risk CV complications [135-138].
Figure 4 reports the basic mechanisms shaped “impaired immune phenotype” of the cells, which secret MVs in peripheral blood. It seems to be that not just epigenetically transformed mature endothelial cells are able to release of MVs, but progenitor endothelial cells can have similar capability also. There is suggestion that mature endothelial cells can be under various epigenetic stimuli (ischemia/hypoxia, increased glucose level, inflammatory cytokine and lipid abnormalities) and they turn into “functionally incompetence” associated with worsening secretom. All these lead to altered signaling between mature cells and progenitor cells and thereby ascertain a mechanism of shaping altered vascular reparation, which in not able counteract microvasculature damages [139-145].
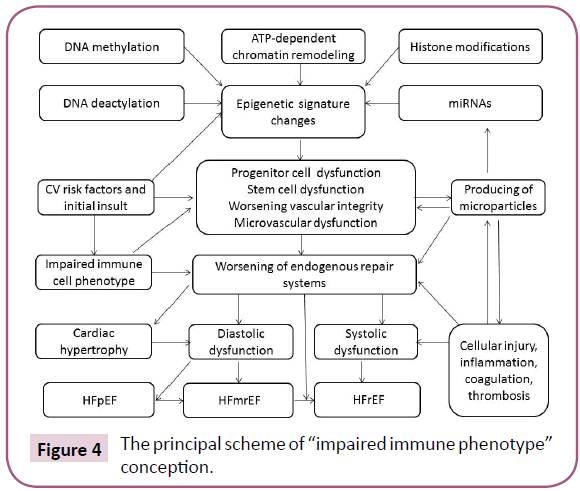
Figure 4: The principal scheme of “impaired immune phenotype” conception.
Thus, in HF pattern of EMVs coordinates an impact of conventional CV risk factors (i.e. insulin resistance, inflammation, thyroid dysfunction, T2DM, abdominal obesity) on vascular reparation and thereby plays a pivotal role in developing endothelial dysfunction that is a central player in HF advance [146-148].
Diagnostic and predictive value of circulating MVs in several phenotypes of HF
Signature of circulating MVs reflecting various stage of pathogenesis of HF could be a biomarker of a risk and severity of HFrEF/HFmrEF and probably HFpEF [20]. Bank et al. [149] reported that both MVs-counts and MVs-content may be novel biomarkers with diagnostic and predictive values for HF. Recently numerous MVs with different origin are detecting to improve conventional risk scores of HF, whereas number and immune phenotypes of EMVs appear to be more promising today. There is evidence that elevated levels of CD62+ MVs, which are secreted with activated mature endothelial cells, may elucidate rather endothelial dysfunction, but lowered number of CD62+ MVs is reported as a marker of severe endothelium injury [21,150].
The concept of “Impaired” Immune phenotype of circulating EMVs as HF biomarker
Altered ratio between numbers of circulating EMVs with different origin is known as impaired phenotype phenomenon. Recently it was found in individuals with endothelial dysfunction and it correlated well with severity of vascular damage [151-154]. Some studies confirmed the idea that simple signature of MVs does not correspond to the number of CV risk factors and that circulating number of single MVs does not adequately elucidate a risk of vascular event and vascular complications [144,155]. The number of apoptotic EMVs alone and adjusted to number of mononuclear progenitor cells shown a much more probability and discriminate value for HF clinical outcomes compared to NT-proBNP, soluble ST2 and galectin-3 used alone [156,157]. All these data led to new predictive score based on serial measure of circulating biomarkers including EMVs, NT-proBNP, soluble ST2 and galectin-3 [158,159]. Thus, “impaired immune phenotype” of circulating EMVs as well as increased number of apoptotic cellderived MVs are novel biomarkers of HF with possible predictive capabilities.
Conclusion
There is gaining interest in the scientific community toward the role of MVs in pathogenesis of HF. MPs are discussed a mediator of several biological processes affecting growth, differentiation, and proliferation of tissues, as well as MVs are essential for immune response, regulation of angiogenesis, neovascularization, and coagulation. There is large number of evidence that number and immune phenotypes of circulating MVs originated from numerous circulating cells (erythrocytes, monocytes, platelets, endothelial cells and tumor cells) could be markers of severity of vascular injury, and endothelial dysfunction. Moreover, there are data received from clinical trials that confirm a participation of EMVs in vascular reparation. Therefore, altered immune phenotype of circulating EMVs associated with increased number of apoptotic cell-derived MVs was found a predictor of CV events and CV disease in patients with HF. Although EMVs are promising biomarkers in HFrEF, HFmrEF and HFpEF, large clinical trials are needed to be confirmed the probability of number and altered immune phenotype EMVs in HF.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Funding and Grants
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interests
Not declared