Research Article - (2023) Volume 9, Issue 1
Received: 02-Jan-2023, Manuscript No. IPBM-22-15246; Editor assigned: 04-Jan-2023, Pre QC No. IPBM-22-15246 (PQ); Reviewed: 18-Jan-2023, QC No. IPBM-22-15246; Revised: 23-Jan-2023, Manuscript No. IPBM-22-15246 (R); Published: 30-Jan-2023, DOI: 10.35841/2472-1646.23.09.001
Pollution of water systems is a potential threat to human health as pollutants absorbed by primary sources in the food chain bio-accumulate. The aim of this study was to determine the impact of coal effluent on the oxidative stress status of aquatic plants. Water and plant samples were collected from four different sites in a coal mining area during the wet and dry seasons. Physical parameters of the water samples were measured on site. Effluent from an underground acid rock drainage point exhibited low pH and high levels of TDS and conductivity. Plants were analyzed for copper, zinc, cadmium and lead. High concentrations of lead and zinc were observed in plants sampled from all sites. Significantly higher metal concentrations were observed during the wet season than the dry season. Plant homogenates were used to determine the activities of superoxide dismutase, catalase, glutathione peroxidase and ascorbate peroxidase. Significantly higher enzyme activities were noted in plants sampled during the wet season compared to plants collected during the dry season. Continual alterations of antioxidant enzymes suggest the potential use of the plants for measuring the effects of coal mining activities. We also observed that the antioxidant systems of aquatic plants are well equipped to deal with oxidative stress induced by coal mining activities.
Oxidative stress; Aquatic plants; Coal mining; Heavy metals; Antioxidant enzymes
Mining largely affects surface and groundwater supplies. The wastewater discharged from coal mines into streams and rivers can compromise water quality, geochemistry, and metal contamination levels, causing a long distance impact on the ecosystem [1,2]. The matrix of natural waters, such as the surface fresh-waters, contains extremely complex and dynamic chemical and biological systems. Usually, several chemical reactions occur between these components, including the dissolution of trace amounts of heavy metals in the water as well as their deposition in underlying sediments. The distribution of heavy metal contaminants within a water body and its components, determines the geochemical and biological reactivity of the aquatic system [3]. Trace metals can be accumulated in the body of the biota and finally end up in the food chain and affect human health [4]. There are certain aquatic species, such as amphipods which possess internal systems that help them maintain the intake of contaminants [5]. Literature shows that there is a constant accumulation of trace metals in these species, irrespective of the available trace metals in the water system [5].
Toxic elements can affect a long list of physiological and biochemical processes in plants and their toxicity varies with plant species, the particular metal, the toxic element concentration and its chemical form [6]. Elevated antioxidant enzyme activity and high contents of non-enzymatic components are important defense mechanisms for plants to tolerate the stress induced by toxic elements. Active oxygen species produced during stress can damage many cellular constituents, including carbohydrates, lipids, proteins and nucleic acids. Research has shown that tolerance of plants to the toxic elements is stimulated by increasing amounts of antioxidants and activity of radical scavenging enzymes [7]. When plants are subjected to environmental stresses, oxidative damage may result because the balance between the production of Reactive Oxygen Species (ROS) and their detoxification by the anti-oxidative system is altered [8]. Antioxidant enzyme activity provides an insight on the extent of adaptation by an organism to pollutant exposure. The current study was carried out to assess the effect of coal mining effluent on the environment by determining the extent of oxidative stress in aquatic plants.
Sampling and Physical Parameter Analysis
Water and plant samples (Bulrush typha) were collected in triplicate from four sampling points and these were assigned as follows; Site A was effluent from an open cast mine, Site B was upstream of a coal processing plant, Site C was underground acid rock drainage point and Site D was effluent from a power production plant (Figure 1). One liter glass reagent bottles were used to collect water samples, whilst new polythene bags were used for collecting sediment and plant samples. Sampling was carried out during the wet and dry seasons. Municipal water from Hwange was used as a control. Temperature, pH, conductivity and total dissolved solids parameters were measured at each site.
Heavy metal analysis in plants: Fresh leaves and stems of plants collected per site were ground separately and two grammes were weighed out and placed in 50 ml beakers containing 5 ml of 55% HNO3. The samples were allowed to stand overnight. The samples were then heated carefully on a hot plate at 80°C for an hour until the production of red NO2 fumes had ceased. The temperature was increased to 120°C and samples were heated for another hour. One milliliter of H2O2 was added and heated for an hour at 120°C. Samples were allowed to cool and 1 ml of 32% HCl was added. The digests were filtered using ashless Whatman 40 filter papers and made up to 50 ml using distilled water [9].
Biochemical Analysis
Leaves and stems of each plant were homogenized in 50 mM sodium phosphate buffer, pH 7.0, containing 1 M NaCl, 1% polyvinylpyrrolidone and 1 mM EDTA and centrifuged at 20,000 g for 15 minutes according to the method of Boojar, et al. (2011) [10]. The Post Mitochondrial Fraction (PMF) was stored at -80°C until required. Protein content of the PMF of the plant homogenates was determined using the method of Lowry, et al. (1951) [11]. The post-mitochondrial fractions were used to determine superoxide dismutase, catalase, glutathione peroxidase and ascorbate peroxidase activities. Plant samples analysed were collected from Sites A, B, C and D (Figure 1).
Figure 1: Map showing sampling collection sites in Hwange
Superoxide dismutase activity: The activity of superoxide dismutase (SOD) in the post-mitochondrial fractions was determined following the method of Sun et al. (1988) [12]. The reaction mixture contained 0.5 ml sample or standard copper, zinc superoxide dismutase (Cu, Zn-SOD) and 2.45 ml SOD Assay Reagent (SODAR) The SODAR contained 40 ml of 0.3 mM xanthine, 20 mL of 0.6 mM ethylenediaminetetraacetic acid (EDTA), 12 mL of 400 mM sodium carbonate, 6 mL of 0.1% w/v bovine serum albumin and 20 mL of 150 μM nitroblue tetrazolium. The working range for the Cu, Zn-SOD standard curve was 0 ng/ml- 300 ng/ml. One enzyme unit of SOD was defined as the amount, which inhibited the nitroblue tetrazolium reaction by 50%. Copper Zn-SOD activity in the samples was calculated as units/mg protein by comparison to the standard curve.
Catalase activity: The activity of catalase was assayed according to the method of Clairborne (1979) [13]. Briefly, the following reagents were added to test tubes: 2950 μl of 19 mM H2O2 solution in 50 mM potassium phosphate buffer (pH 7.0) and 50 μl of 1 mg/ml protein to bring the final volume to 3000 μl. The blank contained 3000 μl of buffer with no substrate. The rate of decrease in absorbance was monitored on a UV/VIS spectrophotometer (240 nm, 25°C). Each sample was assayed in triplicate.
Glutathione peroxidase activity: The activity of glutathione peroxidase (GPx) was assayed following the method of Flohe and Gunzler (1984) [14]. The assay mixture contained 100 μl of 0.1 M potassium phosphate buffer (pH 7), 10 μl of sodium azide, 20 μl of glutathione reductase, 20 μl of reduced glutathione, 20 μl of NADPH in 0.1% NaHCO3, 1.5 mM H2O2 and 10 μl of 1 mg/ml homogenate. The reaction mixture was incubated at 25°C and the decrease was followed at 340 nm using a microtitre plate reader. The extinction coefficient of 6.22 μM-1cm-1 was used.
Ascorbate peroxidase activity: Ascorbate peroxidase (APX) activity was determined according to Nakano and Asada (1981) [15]. Ascorbate peroxidase activity was estimated in 3000 μl assay mixtures that contained 50 mM phosphate buffer (pH 7.0), 0.5 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2 and 25 μl enzyme extract. The decrease in ascorbate concentration was followed at a wavelength of 290 nm for 1 minute and activity was calculated using the extinction coefficient of 2.8 mM cm-1 for ascorbate.
Statistical Analysis
Data were reported as means ± SD of enzyme activities of specific antioxidant enzymes. Two-way ANOVA was used to assess statistical differences among and sites using the GraphPad prism 8 program.
Physico Chemical Analysis of Water Samples
Table 1 shows the physico-chemical results of water samples. Water collected from Site C (underground acid rock drainage point) had high conductivity and total dissolved solids whilst showing low pH compared to other sites and the control for both the dry and wet seasons. The pH of water from Sites A (effluent from open cast mine), B (upstream of coal processing plant), D (effluent from a power production plant) and the control ranged from 5.77-6.56 for the dry season and 7.07-8.32 for the wet season. Significant differences in conductivity and total dissolved solids values were observed between the control site and all sites for both the dry and wet seasons.
|
pH | Temperature (°C) | Conductivity (µS/cm) | Total Dissolved Solid (ppm) | ||||
|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| Control | 5.84 ± 0.0 | 7.12 ± 0.12 | 23.7 ± 0.29 | 31.7 ± 0.1 | 64.9 ± 7.6 | 64.8 ± 0.14 | 32.4 ± 4.2 | 35.1 ± 4.0 |
| Site A | 6.56 ± 0.47 | 8.14 ± 0.02 | 14.2 ± 0.22 | 16.8 ± 0.0 | 1170 ± 73.0 | 795 ± 6.4 | 554.5 ± 0.5 | 398 ± 2.0 |
| Site B | 6.12 ± 0.77 | 7.07 ± 0.18 | 19.6 ± 0.64 | 20.2 ± 0.0 | 309 ± 5.7 | 291.3 ± 19.1 | 158 ± 5.9 | 145.3 ± 9.5 |
| Site C | 2.13 ± 0.14 | 1.79 ± 0.16 | 16.6 ± 0.3 | 16.6 ± 0.3 | 2410 ± 16.3 | 2350 ± 149.9 | 1200 ± 20.0 | 1125 ± 5.0 |
| Site D | 5.77 ± 0.01 | 8.32 ± 0.09 | 22.4 ± 0.29 | 20.7 ± 3.4 | 253 ± 2.5 | 336.3 ± 13.9 | 127.5 ± 0.5 | 172.7 ± 6.1 |
Table 1: Physico chemical parameters of water samples.
Heavy Metal Analysis of Plant Samples
Results of heavy metals obtained from plant samples collected from different sites are shown in Table 2. Higher levels of lead (Pb) and zinc (Zn) were observed for all sites during both the dry and wet season compared to World Health Organization (WHO) and Food and Agriculture Organization (FAO) guidelines for plants. High metal concentrations of copper (Cu), lead (Pb), zinc (Zn) and cadmium (Cd) were observed for plant samples collected from Site C (effluent from underground acid rock drainage point) in the dry season compared to the other sites. Significantly high Zn and Cd concentrations were observed for plants collected from Site D (effluent from power production plant) during the wet season compared to other sites.
| Heavy metal | Sampling site (mg/kg) | WHO Guidelines | |||||||
|---|---|---|---|---|---|---|---|---|---|
| *Site A | *Site B | *Site C | *Site D | ||||||
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | ||
| Cu | 16 ± 0.2 | 18.2 ± 0.2 | 21.7 ± 0.5 | 22.5 ± 0.6 | 25 ± 0.2 | 26.3 ± 0.3 | 20 ± 0.4 | 32.6 ± 0.4 | a10 mg/kg |
| Pb | 103.6 ± 0.6 | 36.6 ± 0.6 | 91.2 ± 5.4 | 285.6 ± 3.4 | 68.2 ± 0.5 | 113.6 ± 3.1 | 141.6 ± 0.8 | 118.8 ± 0.4 | a 2 mg/kg |
| Zn | 570 ± 5.7 | 660 ± 9.9 | 540 ± 6.5 | 710 ± 13.5 | 530 ± 8.0 | 550 ± 20.4 | 600 ± 0.0 | 3740.0 ± 18.7 | b27.4 mg/kg |
| Cd | 2 ± 0.1 | 2.5 ± 0.1 | 3 ± 0.1 | 1.6 ± 0.1 | 4.4 ± 0.0 | 1.9 ± 0.0 | 2.1 ± 0.1 | 21.1 ± 0.1 | a0.02 mg/kg |
a Source: WHO (1996); b Source: FAO/WHO (1984); *Sites A=effluent from coal mine, B=effluent from coal battery, C=effluent from underground acid rock drainage point and D=effluent from power production plant.
Table 2: Heavy metal analysis of plant samples.
Biochemical Analysis
Superoxide dismutase activity: Figure 2 shows the results of superoxide dismutase activity of plants collected from different sites during the dry and wet seasons. There were statistically significant different decreases (p<0.05) in enzyme activities in plants collected from all sites compared to the controls in both during the dry and wet seasons. Superoxide dismutase (SOD) activities of plants collected in the wet season were significantly higher (p<0.05) compared to enzyme activities of plants collected in the dry season. No statistically significant differences in enzyme activities were observed in plants collected from Sites A (effluent from open cast mine), B (upstream of coal processing plant) and C (effluent from underground acid rock drainage point) for both the dry and wet seasons.
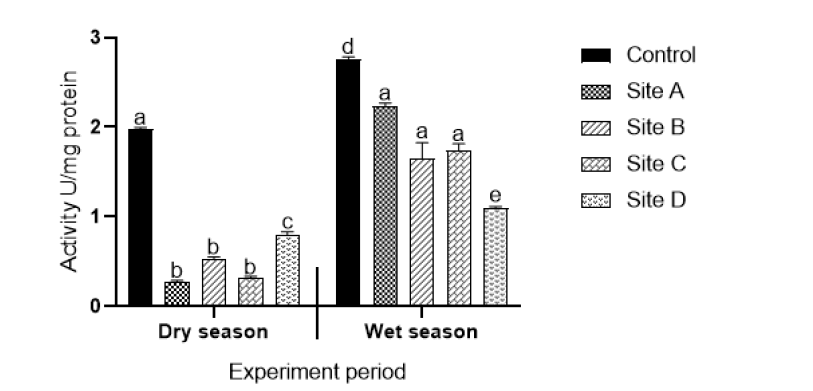
Figure 2: Superoxide dismutase activity of aquatic plants exposed to water from Sites A (effluent from coal mine), B (effluent from coal battery), C (underground acid rock drainage point), D (power production plant). Values are an average representative of triplicate exposures, expressed as mean ± SD. Different letters on bars indicate significant differences (p<0.05) and the same letters on bars indicate that there are no significant differences.
Catalase activity: Results of catalase activity of plants collected from different sites during the dry and wet seasons are shown in Figure 3. There were no statistically significant different changes in catalase (CAT) activities in plants collected during the dry season from all sites when compared to the enzyme activities of plants collected from the control site. Statistically significant increases (p<0.05) in CAT activities were observed in plants collected from Sites A (effluent from open cast mine), C (effluent from underground acid rock drainage point) and D (effluent from power production plant) compared to those of plants collected from the control site during the wet and the dry seasons. No statistically significant different changes in CAT activities were observed in plants collected from the control site during both the dry and wet seasons.
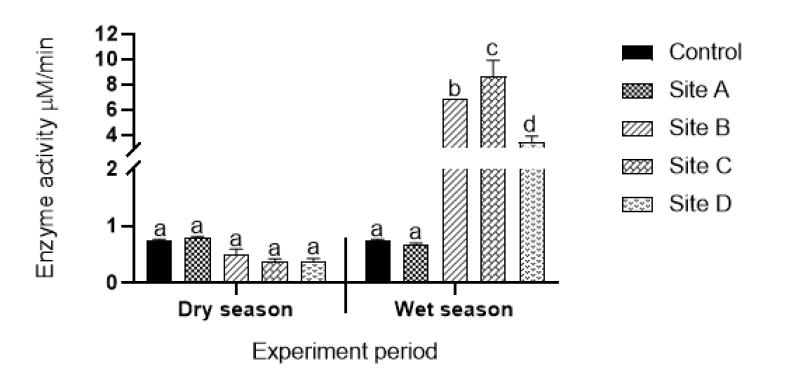
Figure 3: Catalase activity of aquatic plants exposed to water from Sites A (effluent from coal mine), B (effluent from coal battery), C (underground acid rock drainage point), D (power production plant). Values are an average of triplicate exposures, expressed as mean ± SD. Different letters on bars indicate significant differences (p<0.05) and the same letters on bars indicate that there are no significant differences.
Glutathione peroxidase activity: Glutathione peroxidase (GPx) enzyme activity of plants collected from different sites during the dry and wet seasons are shown in Figure 4. No statistically significant different changes in enzyme activities were observed for plants collected during the dry season when compared to enzyme activities in plants collected during the wet season. Statistically significant different increases (p<0.05) in GPx activities were observed for plants collected from all the sites during the wet season compared to enzyme activities in plants collected from the control site. Plants collected from Sites A (effluent from open cast mine) and B (upstream of coal processing plant) exhibited higher GPx activities compared to GPx activities in plants collected from Sites C (effluent from underground acid rock drainage point) and D (effluent from power production
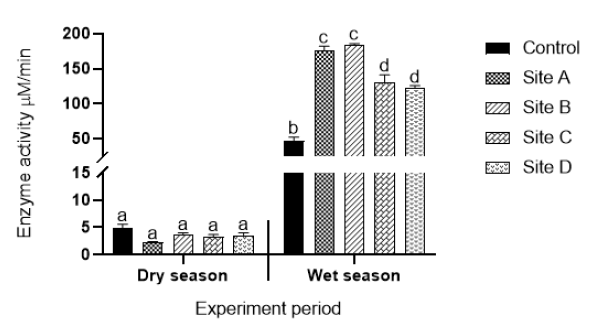
Figure 4: Glutathione peroxidase activity of aquatic plants exposed to water from Sites A (effluent from coal mine), B (effluent from coal battery), C (underground acid rock drainage point), D (power production plant). Values are an average of triplicate exposures, expressed as mean ± SD. Different letters on bars indicate significant differences (p<0.05) and the same letters on bars indicate that there are no significant differences.
Ascorbate peroxidase activity: Figure 5 shows ascorbate peroxidase (APX) activities of plants collected from different sites during the dry and wet seasons. Statistically significant decreases in APX activities were observed in plants collected from all sites compared to enzyme activities in plants collected from the control sites. However, no statistically significant differences in enzyme activity were observed amongst these plants during the dry season. Significant differences in peroxidase enzyme activities were observed in plants collected from Sites A (effluent from open cast mine) and D (effluent from power production plant) compared to enzyme activities in plants collected from Sites B (upstream of coal processing plant) and C (effluent from underground acid rock drainage point) during the wet season. However activity in plants collected from Sites A and D was lower compared to enzyme activities in plants collected from Sites B and C.
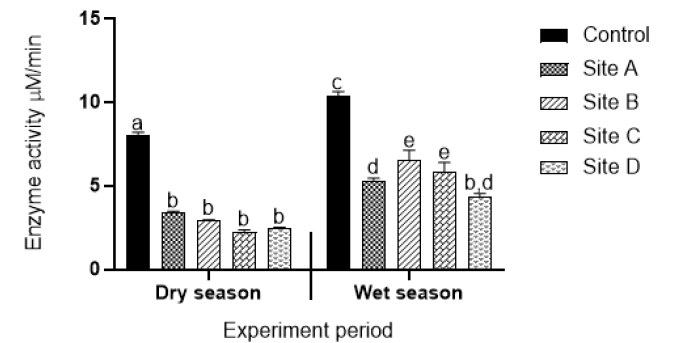
Figure 5: Ascorbate peroxidase activity of aquatic plants exposed to water from Sites A (effluent from coal mine), B (effluent from coal battery), C (underground acid rock drainage point), D (power production plant). Values are an average of triplicate exposures, expressed as mean ± SD. Different letters on bars indicate significant differences (p<0.05) and the same letters on bars indicate that there are no significant differences.
Electrical conductivity gives an indication of the amount of total dissolved solids in water hence high TDS values correlate with high conductivity in water [16]. A high value of total dissolved solids and a high value of Electrical Conductivity (E.C.) of water was observed for water sampled from Site C (underground acid rock drainage point) (Table 1). The E.C. values observed for this site (2410.0 ± 16.3 and 2350.0 ± 149.9 for the dry and wet season respectively) were way above the WHO set standard of 1500 μS/cm (WHO, 2010). The TDS and E.C values of surface water collected from Sites A, B and D were in the ranges of 127.5 ± 0.5 to 554.5 ± 0.5 ppm and 253.0 ± 2.5 to 1170.0 ± 73.0 μS/cm, respectively, compared to control municipal water, which had ranges of 32.4 ± 4.2 to 35.1 ± 4.0 ppm and 64.8 ± 0.14 to 64.9 ± 7.6 μS/cm respectively. In a study of physiochemical parameters to evaluate the drinking water quality by Rahmanian, et al. (2015) in Malaysia, they reported 37 ppm in their control tap water [17]. The TDS value of municipal water observed in our study, an average of 34 ppm, is comparable to this. In contrast, the average conductivity value of 64.85 ± 5.37 μS/cm observed for the control municipal water in the current study was lower than the conductivity for the tap water assayed in Malaysia, which ranged from 78.8 μS/cm-95.0 μS/cm.
The World Health Organisation recommends a pH value of 6.5 or higher for drinking water [18]. Values lower than the stipulated pH value of 6.5 (WHO, 2010) were observed for water samples collected from the Control site, Sites B, C and D (Table 1) [18-20]. In our study, a pH average of 1.96 ± 0.15 was measured for water samples collected from Site C, (acid mine drainage site) which is lower than the pH range of 3.2-4.6 observed in the studies by Campaner, et al. (2014) [19]. For acid mine drainage in Southern Brazil. Furthermore, the river and groundwater samples of municipal public water supplies in Southern Brazil revealed a pH range of 7.2 to 7.5, which is higher than the range of 5.84 to 7.12 observed for the control municipal water in the our study. The pH of surface waters is important to aquatic life because pH affects the ability of fish and other aquatic organisms to regulate basic life-sustaining processes, primarily the exchanges of respiratory gases (Robertson-Byron Inc. 2004) [20]. Our results showed a pH range for surface water of 6.12 to 8.32 which compares well with the pH of 6-10.8 for surface waters observed by Campaner et al., (2014) [19]. Acid mine drainage caused significant changes to the physico-chemical parameters of water.
Aquatic plants can take up large amounts of metals from water and/or sediment through active and/or passive absorption. This is achieved through different organs such as roots, stems, and leaves. Metals, like lead, may be introduced into aquatic ecosystems as effluent from mining and industrial activities. Literature shows that metals induce oxidative stress in cells of living organisms which results from an imbalance between the generation and elimination of reactive oxygen species [21,22]. In our study, the order of metal concentration in aquatic plants was Zn>Pb>Cu>Cd with plants collected from Site D (effluent from power production plant) in the wet season exhibiting high concentrations for all metals (Table 2). The uptake of metals, as well as the degree of tolerance of pollutants by plants is dependent on the bioavailability of the metals, plant species and their metabolism [23-25].
Plants exposed to heavy metals have been shown to produce increased amounts of ROS [26-30]. Chronic exposure to Cu Zn, Pb and Cd induce oxidative stress by generating toxic oxygen derivatives and alter the activity of the antioxidant defence system in plants and leads to oxidative damage [18-20]. Increased levels of Zn and Pb have been shown to induce oxidative stress in Hibiscus esculentus plants [22]. A study by Toteja et al., (2017) showed that SOD is induced in the presence of both Cd and Cu [31]. In contrast, increased levels of Cu caused decreases in SOD activity in our study. This decrease in SOD activity can be attributed to the inactivation of the enzyme by H2O2 formed as a result of metal exposure. SOD catalyses superoxide radicals to molecular oxygen and hydrogen peroxide [32]. However, we also observed an increase in SOD activity in the wet season when compared to the dry season. This increase can be attributed to increased salinity and metal contamination. This is in agreement with increases in SOD activities reported by Youssef and Azooz, 2013 in okra [33-35]. Induction of catalase, glutathione peroxidase and ascorbate peroxidase (APX) activities were observed in the current study for the wet season compared to the dry season (Figures 2-4). This may be as a result of Zn and Pb concentrations which were generally higher during the period. Again, this is in agreement with the findings of Youssef and Azooz, (2013), who observed induction of CAT and APX activities as a result of Zn and Pb stress of the Hibiscus esculentus plants [36,37]. Lead exposure has been shown to elevate APX, CAT and SOD enzyme activity in hyacinth seedlings, with APX showing a positive correlation with an increase in lead concentration to maintain the balance between the formation of ROS and their removal [26-30]. When there is an inadequate response of CAT activity, there is an induction of APX to compensate for the reduced activity of CAT [31]. Increases of GPx activities were observed for plants collected from all the studied sites (Figure 3) and the results are in agreement with the study of Vestena, et al. (2011) who showed an increase in GPx activity in the plant Salvinia auriculata exposed to Cd [38-40]. Also this induction of GPx when CAT activity is reduced has been reported in some animals, for example, freshwater snails [Our study shows that pollution, due to heavy metals, causes enhancement of GPx activity which detoxifies peroxides in various cells and tissues of Bulrush typha. Overall these results demonstrate that an additive effect of the heavy metals and physico-chemical parameters in the induction of GPx activity in plants [41-43].
We can conclude that the combination of the TDS, conductivity, pH and heavy metals regulate SOD, CAT, GPx and APX enzyme activities.
This work was supported by funds from International Science Program, Uppsala University Sweden. Support from the Ecotoxicology Research Group and Applied Biology and Biochemistry Department of the National University of Science and Technology Zimbabwe was much appreciated.
The authors have no conflict of interest to report.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Change JB, Siwela AH, Basopo N (2023) Oxidative Stress Associated with Pollutants from Coal Mining Activities on Aquatic Plants: A Case Study of Hwange, Matabeleland North, Zimbabwe. Biomark J. 9:001.
Copyright: © 2023 Change JB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.