Keywords
EDTA; Endothelial cells; Biodistribution
Abbrevations
EDTA: Ethylene Diamine Tetra Acetate; HUVEC: Human Umbilical Vein Endothelial Cell; TNFα: Tumor Necrosis Factor Alpha; CVD: Cardio Vascular Diseases; ND: Neurodegenerative Diseases; Cd: Cadmium; As: Arsenic; Pb: Lead; VEGF: Vascular Endothelial Growth Factor; MAPK: Mitogen-Activated Protein Kinase; Fe: Iron; CNS: Central Nervous System, Al: Aluminum; Cu: Copper; Cr: Chromium; Hg: Mercury; ROS: Reactive Oxygen Species; 99mTc: Technetium-99m; 99Mo: Molybdenum-99; TcO4 -: Pertechnetate; CaNa2: EDTA Calcium Disodium EDTA; PBS: Phosphate Buffered Saline; Ig-FITC: Immunoglobulin- Fluorescein Iso Thio Cyanate; SPET: Single-Photon Emission Computed Tomography; ID: Injected Dose; Mn: Manganese; MnDPDP: Mangafodipir Trisodium
Introduction
EDTA (ethylenediaminetetraacetate) chelation therapy can be used for the treatment of cardiovascular diseases (CVD) [1,2] and neurodegenerative diseases (ND) [3,4] due to its ability to remove the toxic metals that are responsible for damage to both endothelial cells and neurons. The toxic metals cadmium (Cd), arsenic (As), and lead (Pb) are able to target the vascular system in a variety of ways, ranging from hemorrhagic injury to subtle pathogenic remodeling and metabolic changes in specific organs [5]. In particular, Cd at high concentrations damages human umbilical vein endothelial cells (HUVEC), disrupts tube formation, and inhibits vascular endothelial growth factor (VEGF) expression and the activity of mitogen-activated protein kinase (MAPK) pathways, thus interfering with VEGF-mediated angiogenesis [6]. In vitro studies on endothelial cells show that iron (Fe), in combination with palmitic acid, potentiates its toxicity, as seen in mitochondrial dysfunction, cell death, apoptosis and DNA mutation [7]. Exposure to As, Cd, and Pb mixtures impairs myelin and axon development in the rat brain, optic nerve, and retina [8]. The neurotoxic effects of Cd possibly derive from the association of both biochemical changes in the cells and functional changes in the central nervous system (CNS), especially after long-term Cd exposure [9]. In addition, Cd causes, in a dose and timedependent manner, significant lesions to the peripheral auditory nerve fibers, spiral ganglion neurons, and sensory hair cells in organotypic cultures obtained from postnatal rat cochleae [10]. The neurotoxicity of aluminum (Al) [11], and in vivo evidence for altered hippocampal volume and brain metabolites in workers occupationally exposed to Pb (using magnetic resonance imaging) have already been described [12]. The dangerous effects of toxic metals have been associated with their induced oxidative stress on cells. Redox-active metals, such as Fe, copper (Cu), and chromium (Cr) undergo redox cycling, whereas redoxinactive metals, such as Pb, Cd, and mercury (Hg) deplete major antioxidants within cells, especially thiol-containing antioxidants and enzymes [13]. Either redox-active or redox-inactive metals may cause an increase in production of reactive oxygen species (ROS).
Atherosclerosis is a chronic progressive vascular disease. Functional impairment of the endothelium (endothelial dysfunction) is one of the first recognizable signs of atherosclerosis, and is present long before the occurrence of atherosclerotic CVD [14]. Since endothelial activation represents the pathogenetic mechanism of the atherosclerotic process, factors promoting endothelial dysfunction (or “activating factors”), when identified, might be attenuated by suitable therapeutic treatment. EDTA, administered intravenously to patients, is able to remove toxic metals from vessels: indeed, the damage caused to endothelial cells by these metals might be avoided with the use of chelation therapy. We aimed to highlight the effects of EDTA on endothelial cells, by studying the in vitro efficacy of EDTA treatment to control HUVEC activation induced by a proinflammatory cytokine. Furthermore, as some authors have stated that EDTA is unable to cross the blood brain barrier and remove toxic metals from the CNS, we studied in vivo (rats) the distribution of intravenously injected radiolabeled EDTA.
Materials and Method
Chemicals used for EDTA radiolabeling
Unless otherwise noted, all chemicals were of reagent grade. The [99mTc] complexes were prepared from ‘no carrier added’ [99mTcO4 -] obtained by elution of a commercial [99Mo / 99mTc] generator with normal saline.
A fresh solution of [99mTc]TcO4 - (555 MBq/ml) was prepared by elution of a GE Healthcare Drytec 12 GBq generator; simultaneously a solution of SnCl2 (1 mg/ml) was prepared by dissolving 10 mg of Tin (II) Chloride (Sigma Aldrich Chemistry, Milwaukee, WI, USA) in 10 ml of Water For Injection. A solution of EDTA was then prepared (10 mg/ml) by dissolving 100 mg of EDTA in 10 ml of Water for Injection.
The labelling reaction was performed by mixing in a vial: 1 ml of EDTA solution containing 10 mg/ml of calcium disodium EDTA (CaNa2 EDTA), 1 ml of fresh solution of [99mTc] TcO4 - containing 555 MBq/ml, and finally 0.5 ml of SnCl2 containing 1 mg/ml. After gentle mixing, 0.5 ml of the final solution was drawn into the syringe used for rat administration.
Quality control was performed with Thin Layer Chromatography, using silica gel (ITLC SC Gelman Science, Ann Arbor, MI, USA) as a stationary phase and two different mobile phases: a solution obtained by mixing a saline solution (0.9%) with water (1/1, V/V); methyl-ethyl-ketone (Sigma Aldrich Chemistry) 99.9%. After development (approximately 10 minutes), chromatograms were analyzed and integrated using apparatus equipped with a radiometric detector and a dedicated personal computer (Raytest MiniGita, Elysia Germany GmbH, Straubenhardt, Germany). Purity was always > 90%.
Animals
Healthy 230 – 250 g adult male Wistar rats (Harlan Italy, S.Pietro al Natisone, Udine, Italy) were used. The rats had free access to standard pelleted food and water, and were maintained at a temperature of 22 ± 1°C with a 12 h light / dark cycle. All experimental procedures conformed with the guide for the care and the use of Laboratory Animals published by the US National Institute of Health (NIH publication NO: 85 - 23, revised 1996), in accordance with the animal welfare regulations of Italian local authorities (Ethics Committee of the Università degli Studi di Milano, Italy).
Evaluation of biodistribution [99mTc] EDTA
Labeled EDTA distribution was time evaluated (for 3h), using a triple-head SPET gamma camera (IRIX Philips) equipped with high-resolution, low-energy collimators (Picker-Prism 3000, Cleveland, Ohio, USA). Image acquisition was performed with a single head, setting the energy window at 126 - 154 keV, in 256 × 256 matrix (pixel size = 1.167 mm) and a duration of 60s. During the procedure, the animals were briefly anesthetized and immobilized. Between each scan, the rats were allowed to stay in the cage.
Autopsy
The animals were sacrificed under ether anesthesia at 1 hour or at 3 hours after labeled EDTA injection. The rats were dissected, and the organs of interest were weighed and placed in specific containers to be measured using the gamma counter. Radioactivity measurement in each organ was carried out using a Wallac Wizard gamma counter (Wallac Oy, 20101 Tunka, Finland). In each sample, cpm were measured over 120 seconds and the measurement of the blank sample (empty container) was subtracted from these values. Samples of muscle, brain, heart, lung, liver, spleen, kidney, and intestine were obtained and weighed, and specific EDTA activity [99mTc] was determined. Following this, the activity of EDTA [99m Tc] per gram (g) of tissue net weight was calculated. The results for each organ were expressed as percentages of the recovered dose and calculated as follows: (radioactivity recovered from the organ of interest / sum of radioactivity recovered from all organs removed subtracted from the blank) × 100.
Statistical analysis
Statistical analysis was carried out using the Student's t test. Significance was assumed when P < 0.05.
Endothelial cell culture
HUVEC were isolated from human cords by collagenase as described [15] and cultured in 1% gelatin-coated flasks (Falcon, Becton Dickinson, Bedford, MA, USA) using endotoxin-free Medium 199 (BioWhittaker, Cambrex BioScience, Verviers, Belgium) containing 20% heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 1% bovine retinal-derived growth factor, 90 μg/ml heparin, 100 I.U./ml penicillin, and 100 μg/ml streptomycin (Biochrom, Berlin, Germany) (Complete Medium, CM). All experiments were carried out with HUVEC at passage 1 - 4.
HUVEC (1 × 105 cells in CM plated on glass coverslips) were treated with TNFα (200 U/ml/2 hsr) (R&D System, Abingdon, UK) and / or EDTA (10 mg/ml / 2 hours) and fixed with 2% paraphormaldehyde in PBS (phosphate buffered saline) and washed twice in PBS for fluorescence microscopy. After permeabilization (Triton X - 100, BDH, 0.1% / 1 min), HUVEC were labeled with a monoclonal antibody (clone 20C6, dil 1:100) specific for α - tubulin followed by a secondary antibody rabbit anti-mouse Ig-FITC (DAKO, Copenhagen, Denmark) and with phalloidin TRITC that labeled F-actin (Sigma Aldrich). Coverslips were mounted using 50% glycerol in PBS. Microscopic analysis was carried out using a BioRad MRC 1000 confocal scanning microscope (BioRad Laboratories, Milan, Italy) and x 63 lens. Digital fluorescence images were recorded.
Results
EDTA biodistribution [99m Tc-]
A gamma camera was used to follow distribution.
Figure 1 shows the distribution of labeled EDTA and cpm/pixel values found during autopsy at 1 h after intravenous injection. At this time, increased radioactivity was noted in many organs, especially in the kidneys that are the site of radioactivity release.
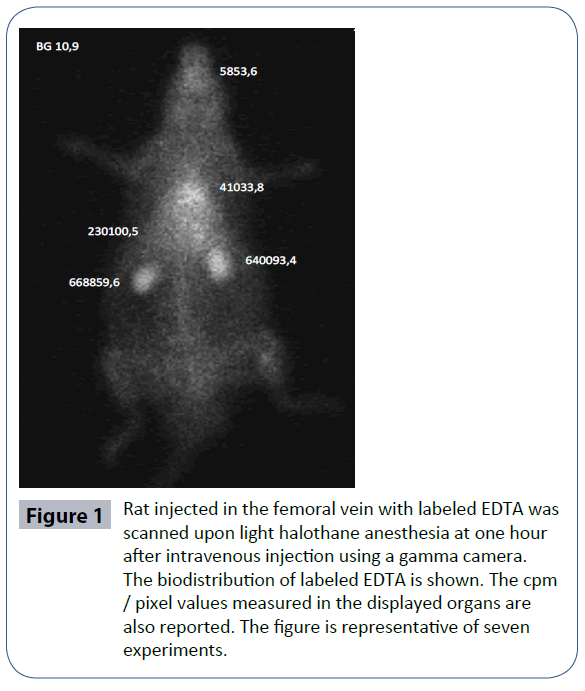
Figure 1 Rat injected in the femoral vein with labeled EDTA was scanned upon light halothane anesthesia at one hour after intravenous injection using a gamma camera. The biodistribution of labeled EDTA is shown. The cpm / pixel values measured in the displayed organs are also reported. The figure is representative of seven experiments.
Autopsy
The rats were sacrificed at 3 h after labeled EDTA injection. Radioactivity of the different organs was calculated separately and expressed per gram of tissue. Labeled EDTA was recovered from kidneys, lungs, CNS, liver, spleen, heart, muscle, and intestine and expressed in % biodistribution of injected dose (ID)/g tissue (Figure 2). Table 1 shows labeled EDTA biodistribution compared with that of [99m Tc] TcO4 - alone. A significant increase in labeled EDTA biodistribution compared with [99m Tc] TcO4 - alone was seen in the CNS, as well as in kidneys. Conversely, distribution of [99m Tc] TcO4 - alone was significantly more evident in the intestine than labeled EDTA.
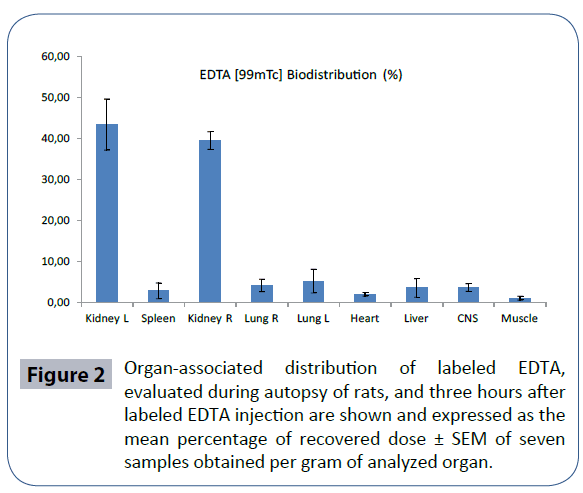
Figure 2 Organ-associated distribution of labeled EDTA, evaluated during autopsy of rats, and three hours after labeled EDTA injection are shown and expressed as the mean percentage of recovered dose ± SEM of seven samples obtained per gram of analyzed organ.
| |
EDTA [99mTc] Biodistribution (%) |
SD |
99mTc Biodistribution (%) |
SD |
| Kidney |
75,87 |
4,19 |
44,07 |
8,39* |
| Spleen |
2,86 |
1,94 |
5,58 |
1,89 |
| Lungs |
9,40 |
2,35 |
8,55 |
4,39 |
| Heart |
2,01 |
1,19 |
0,43 |
0,49 |
| Liver |
3,56 |
0,94 |
2,26 |
2,26 |
| CNS |
3,67 |
0,43 |
0,94 |
0,84* |
| Intestine |
1,30 |
0,46 |
9,40 |
2,21* |
| Muscle |
1,08 |
0,55 |
0,46 |
0,46 |
Table 1: The results obtained at autopsy of rats, performed three hours after injection of labeled EDTA or [99m TcO4-] alone, are reported and expressed as mean ± SEM of seven experiments. * p ≤ 0.05 vs. [99m TcO4-] EDTA.
The radioactivity data obtained at autopsy are in accordance with data obtained using scintigraphy.
The radioactivity data obtained at autopsy are in accordance with data obtained using scintigraphy.
Resistance offered by the vascular endothelium to the uncontrolled transit of macromolecules is guaranteed by close cell-to-cell contact, which in turn is connected with cytoskeletal components. Thus, we investigated the cytoskeletal architecture of HUVEC cultured in vitro with EDTA in comparison with TNFα. As confirmed in Figure 3, TNFα generated F-actin stress fibers along the major axis of the cellular soma, while alpha-tubulin was concentrated in the perinuclear area. Upon EDTA culture, a network-like distribution of bundles was evident, which appeared more subtle and less dense. The position of TNFα and EDTA resulted in the presence of thin and packed peripheral bundles, accompanied by normal tubulin distribution, in keeping with a well spread, quiescent (not activated) phenotype. In Conclusion, in vitro studies showed that EDTA protects endothelial cells against TNFα-induced treatment activation. Such protection might explain the efficacy of EDTA in preventing the in vivo progress of atherogenic processes.
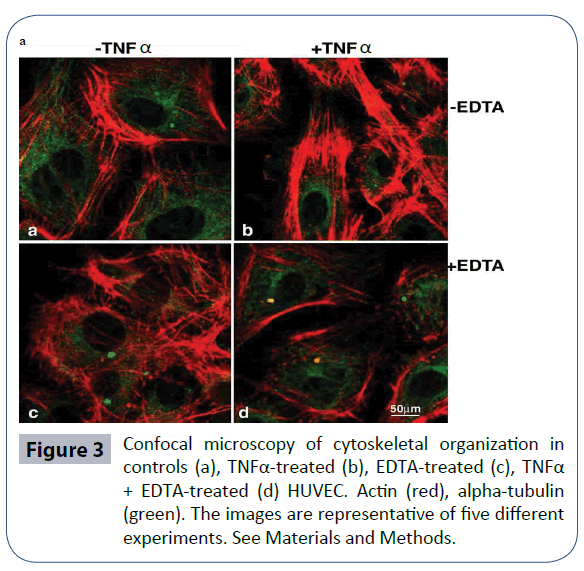
Figure 3 Confocal microscopy of cytoskeletal organization in controls (a), TNFα-treated (b), EDTA-treated (c), TNFα + EDTA-treated (d) HUVEC. Actin (red), alpha-tubulin (green). The images are representative of five different experiments. See Materials and Methods.
Discussion
Manganese (Mn) toxicity was first observed over ten years ago due to its accumulation in the CNS. Manganese is a required element, and a metabolic byproduct, of the contrast agent mangafodipir trisodium (MnDPDP). It is initially sequestered by the liver for first-pass elimination, allowing enhanced contrast in diagnostic imaging [16]. Intravenous Mn administration affects the human body’s homeostatic balance and can lead to toxicity. Occupational, environmental, and medical Mn overexposure can take place. Neurotoxicity, due to the inhalation of airborne Mn, has been noted in miners exposed to elevated concentrations of manganese dioxide, as well as in workers in dry-cell battery factories [17], smelters [18], and welders [19,20]. Manganese neurotoxicity results from accumulation in the brain tissue, and provokes progressive disorders of the extrapyramidal system as occurs in Parkinson’s disease. Manganese can enter the brain along three known pathways: capillary endothelial cells of the blood–brain barrier, the choroid plexus of the blood-CSF (cerebrospinal fluid) barrier, or via the olfactory nerve from the nasal cavity directly into the brain. Toxicity mostly occurs following inhalation exposure. Brain import, with no evidence of export, would lead to Mn accumulation and neurotoxicity [16]. Chelation therapy has been recommended in severe cases of Mn poisoning to reduce the body burden of Mn, and to alleviate symptoms. Follow-up has been carried out on patients, workers who were subsequently removed from exposure, affected by Mninduced Parkinsonism after numerous treatment applications with CaNa2EDTA (2 g in 500 ml physiological saline) [21]. Results confirm that excellent clinical, biological, and neuroradiological data were obtained from chelation therapy associated with timely removal from exposure, and that amelioration persisted over time. Radiolabeling of EDTA-Mn with Tc-99m using a direct labeling method was recently carried out to study its distribution in nude mice [22]. Increased instances of cancer, CVD, and mortality have been associated with exposure to lead (Pb) [23]. The correlation between metal-induced oxidative stress and human disease has been assessed: redox-inactive metals, such as Cd, As, and Pb display their toxic effects via binding to sulfhydryl groups of proteins, and glutathione depletion [24]. Recently, CaNa2EDTA chelation ameliorated the alterations linked to Pbmediated oxidative stress, attenuating cell damage in workers exposed to this toxic metal [25]. We have used chelation therapy with CaNa2EDTA to remove Al intoxication in patients affected by ND [4]. Since chelation therapy has also had beneficial effects on patients affected by CVD [1], in the present study we highlight: i) the effects of CaNa2EDTA (herein referred to as EDTA) on endothelial cells; ii) the possible distribution of the labeled chelating agent at CNS level by observing the biodistribution of [99m Tc] EDTA in the organs of rats following intravenous injection. Our results show that: i) EDTA is able to protect endothelial cells from activation induced by TNFα; ii) distribution of labeled EDTA at CNS level is evident. Vascular endothelial injury is provoked by ROS generated during ischemia, as well as during inflammatory processes. The pro-inflammatory cytokine TNFα contributes to vascular endothelial injury, leading to the endothelial expression of selectins and other adhesion molecules, which enhances leukocyte-endothelial interaction and increases endothelial permeability [26]. Activated endothelial cells release vasoactive substances (nitric oxide, endothelin, platelet activating factor, prostacyclin, mitochondrial N-phormyl peptide), inflammation mediators (TNF, interleukins, interferons), and thrombogenic factors [27]. Endothelial activation represents the primum movens of the atherosclerotic process. EDTA treatment might protect vascular endothelium from TNFα-induced injury. In the present study, we also show that the biodistribution of intravenously injected labeled EDTA in rats was seen in kidneys, lungs, CNS, liver, spleen, heart, and muscle. The uptake of labeled EDTA in the CNS, confirmed by scintigraphy and following dissection of the rat and removal of the brain, proves that EDTA can reach the CNS in these healthy animals.
The results obtained propose new insights into the beneficial effects of EDTA treatment in patients affected by CVD or ND.
Metal pollutants are cardiovascular risk factors, and EDTA chelation therapy might therefore be used to remove some of the causes of CVD and ND.
Acknowledgement
The Authors wish to thank Michael John of the Vita-Salute University in Milan for the English language editing of this paper.
References
- Peguero JG, Arenas I, Lamas GA (2014) Chelation therapy and cardiovascular disease: connecting scientific silos to benefit cardiac patients. Trends Cardiovasc Med 24: 232-240.
- Lamas GA (2015) Cardiology Patient Page. Chelation therapy: a new look at an old treatment for heart disease, particularly in diabetics Circulation 131:e505-e506.
- Fulgenzi A, Vietti D, Ferrero ME(2014) Aluminium involvement in neurotoxicity. Biomed Res Int 2014:758323.
- Fulgenzi A, De Giuseppe R, Bamonti F, Vietti D, Ferrero ME (2015) Efficacy of chelation therapy to remove aluminium intoxication. J Inorg Biochem.
- Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, et al. (2008) The vascular system as a target of metal toxicity. Toxicol Sci 102:207-218.
- Kim J, Lim W, Ko Y, Kwon H, Kim S, et al. (2012) The effects of cadmium on VEGF-mediated angiogenesis in HUVECs. J Appl Toxicol 32: 342-349.
- Yao D, Shi W, Gou Y, Zhou X, Yee Aw T, et al. (2005) Fatty acid-mediated intracellular iron translocation: a synergistic mechanism of oxidative injury. Free Radic Biol Med 39:1385-1398.
- Rai NK, Ashok A, Rai A, Tripathi S, Nagar GK, et al. (2013) Exposure to As, Cd and Pb-mixture impairs myelin and axon development in rat brain, optic nerve and retina. Toxicol Appl Pharmacol 273: 242-258.
- Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxid Med Cell Longev 2013:898034.
- Liu H, Ding D, Sun H, Jiang H, Wu X, et al. (2014) Cadmium-induced ototoxicity in rat cochlear organotypic cultures. Neurotox Res 26: 179-189.
- Exley C (2014) Why industry propaganda and political interference cannot disguise the inevitable role played by human exposure to aluminum in neurodegenerative diseases, including Alzheimer's disease. Front Neurol 5:212.
- Jiang YM, Long LL, Zhu XY, Zheng H, Fu X et al. (2008) Evidence for altered hippocampal volume and brain metabolites in workers occupationally exposed to lead: a study by magnetic resonance imaging and (1)H magnetic resonance spectroscopy. Toxicol Lett 181: 118-125.
- Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1: 529-539.
- Park KH, Park WJ (2015) Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J Korean Med Sci 30: 1213-1225.
- Ferrero E, Ferrero ME, Pardi R, Zocchi MR (1995) The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett, 374: 323-326.
- Crossgrove J, Zheng W (2004) Manganese toxicity upon overexposure. NMR Biomed 17: 544-553.
- Keen CL, Lonnerdal B (1995) Toxicity of essential and beneficial metal ions, Manganese. In: Berthon G, editor. Handbook of Metal-Ligand Interactions in Biological Fluids. Marcel Dekker, NewYork683-688.
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, et al. (1989) Chronic manganese intoxication. Arch. Neurol 46:1104–1106.
- Chandra SV, Shukla GS, Srivastava RS, Singh H, Gupta VP (1981) An exploratory study of manganese exposure to welders. Clin. Toxicol 18: 407-416.
- Ono K, Komai K, Yamada M (2002) Myoclonic involuntary movement associated with chronic manganese poisoning. J. Neurol. Sci 199: 93-96.
- Herrero Hernandez E, Discalzi G, Valentini C, Venturi F, Chio A, et al. (2006) Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology 27:333-339.
- Qi Y, Li G, Chi X, Du L, Huang K, et al. (2015) Preparation of (99m)Tc-EDTA-MN and Its Bioimaging in Mouse Zhongguo Fei Ai Za Zhi 18:422-426
- Lustberg M, Silbergeld E(2002) Blood lead levels and mortality. Arch Intern Med 162:2443-2449.
- Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65-87.
- Cabarkapa A, Borozan S, Zivkovic L, Stojanovic S, Milanovic-Cabarkapa M, et al. (2015) CaNaEDTA chelation attenuates cell damage in workers exposed to lead-a pilot study. Chem Biol Interact 242:171-178.
- Fisher M (2008) Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis 5 Suppl 1:S4-S11.
- Gulati A(2015) Vascular Endothelium and Hypovolemic Shock. Curr Vasc Pharmacol.