Keywords
Neonatal seizure; Rotavirus infection; White matter injury; Diffusion-weighted imaging
Introduction
Seizures are one of the most common, and sometimes the only, distinctive clinical manifestations of neurological dysfunction in neonates [1]. Their incidence is significantly higher among neonates than in individuals of any other age group. Despite improvements in neonatal care, the rates of neurological sequelae after neonatal seizures remain high. Seizure etiology is known to play a primary role in long-term neurodevelopmental outcomes [2]. Viral infection is rare but is a critical cause of seizures that should not be overlooked. Herpes simplex virus, cytomegalovirus, enterovirus, human parechovirus, and rotavirus have been considered important causative viruses in cases of neonatal seizures [3-5].
Rotavirus is the most common cause of gastroenteritis in infants and young children worldwide. In addition, several studies have demonstrated that rotavirus can cause a wide range of neurological manifestations [6,7]. Recently, a few interesting studies have described a distinctive white matter injury associated with neonatal seizures in the context of rotavirus infection [8-10]. Diffusion-weighted images (DWIs) clearly show characteristic findings of extensive and symmetric diffusion restriction in subcortical white matter of the cerebral hemisphere including the corpus callosum [1].
This retrospective study was conducted to describe the brain magnetic resonance imaging (MRI) findings including DWIs, and short-term neurodevelopmental outcomes in infants with neonatal seizures associated with rotavirus infections.
Materials and Methods
Patients
We reviewed the medical records of neonates who were admitted to the neonatal intensive care unit (NICU) of the Inje University Haeundae Paik Hospital from March 2012 to March 2016. Full-term neonates with clinically evident neonatal seizures (within the first 28 days after birth) were considered. The seizures were diagnosed by a neonatologist or pediatrician based on clinical observations using internationally accepted criteria [1]. Infants who tested positive for stool rotavirus antigens and diffusion restriction in cerebral white matter were enrolled [2].
Data collected included information regarding gestational age, birth weight, sex, and mode of delivery, perinatal clinical factor, and Apgar score. Information on electroencephalographic (EEG) findings, neuroimaging findings, and clinical course after admission including response to antiepileptic drugs (AEDs) was also obtained. Perinatal clinical factors including presence of fetal distress, meconiumstained fluid, need of resuscitation in the delivery room, and maternal history were also recorded [3]. Seizure types were categorized in accordance with Volpe’s classification schema, based on the seizure semiology documented in the medical records. The types included subtle, focal clonic, multifocal clonic, myoclonic, and tonic seizures. If multiple types of seizures were recorded, the most prominent one was selected.
EEG and neuroimaging
If a seizure was diagnosed, inter-ictal EEG monitoring was performed as soon as possible in tolerated neonates (Medelec, Oxford, UK). EEG records were reviewed by a pediatric neurologist, and the findings were classified into three groups based on inter-ictal activity: Group 1, normal or mildly abnormal (increased sharp activity, absent or decreased frequency of normal patterns, excessively long low-voltage periods or overall slightly decreased voltage); Group 2, moderately abnormal (moderately abnormal asymmetries in voltage or frequencies, increased asynchrony for age); Group 3, severely abnormal (burst suppression pattern, severe abnormal isoelectric or low-voltage invariant activity, permanent discontinuous activity). Follow-up EEG was performed at 1 and 3 months after discharge. Additional follow-up EEG was performed in accordance with the decision of a pediatric neurologist. Brain MRI was performed on 3.0-T system (Intera Achieva;Phillips, Best, Netherlands). The protocol included Tw-weighted imaging, T2-weighted imaging, fluid-attenuated inversion recovery imaging, and diffusionweighted imaging with preparation of apparent diffusion coefficient maps [4,5].
Follow up and neurodevelopmental outcomes
Neurodevelopmental outcomes were classified as favorable or adverse by our multidisciplinary team based on the available documentation of medical records at last follow-up. Cerebral palsy, global developmental delay (GDD), and epilepsy were classified as adverse outcomes. GDD was defined as a significant delay in two or more developmental categories, including gross and fine motor, speech and language, cognition, personal-social, and activities of daily living.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Sciences software version 18.0 (SPSS Inc., Chicago, IL). The Chi-square and Fisher exact tests were used for categorical variables in group comparisons. Student t-test and Mann-Whitney U-test were used for double comparisons [6]. The results were evaluated with a confidence interval of 95%. P-values less than 0.05 were considered significant.
Results
During the study period, a total 2919 infants were admitted, and 196 full-term infants were diagnosed with seizures. Among 196 infants, 41 infants had positive results in the stool rotavirus antigen test [7]. Of these infants, 26 infants were enrolled who presented with seizures and diffusion-restricted lesions in bilateral cerebral white matter on DWI scans (Figure 1).
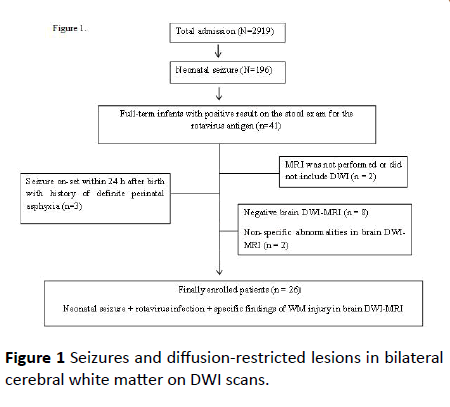
Figure 1: Seizures and diffusion-restricted lesions in bilateral cerebral white matter on DWI scans.
Clinical characteristics and seizure semiology
The retrospective cohort for analysis included 15 (57.7%) boys and 11 (42.3%) girls. The mean birth weight was 3,259 ± 527.9 g (2250.0-4940.0 g) and mean gestational age was 38.6 ± 5 weeks (37+0 to 40+3 weeks). Table 1 shows the clinical characteristics and seizure semiology [8]. All infants were delivered at local obstetric clinics and they were relatively healthy for several days after birth. They were all transferred to our NICU because of seizure. Seizures developed within the first week of life (median 5 days, range 4 - 6 days after birth). Only five infants (19.2%) showed signs of infection at 1-2 days before the seizure on-set, including poor feeding, diarrhea, and mild fever. The most common accompanying symptom was apnea and it was observed in nine infants Seizures were brief and repetitive, occurring more than 10 times in some cases. Focal or multi-focal clonic were the predominant seizure type. Similarly, most infants showed.
| Variables |
Number of infants |
Favorable |
Unfavorable |
p-value |
| Outcome |
Outcome |
| Total |
21 |
5 |
- |
| Gestational age (weeks) |
38.65 ± 0.89 |
38.8 ± 0.52 |
0.74 |
| Birth weight (grams) |
3345.24 ± 530.29 |
2898 ± 365.81 |
0.09 |
| Delivery type |
| Spontaneous |
8 |
6 (75.0) |
2 (25.0) |
0.62 |
| Cesarean delivery |
18 |
15 (83.3) |
3 (16.7) |
| Apgar score at 5 mins |
| <7 |
1 |
1 (100.0) |
0 (0.0) |
0.43 |
| ≥ 7 |
25 |
20 (80.0) |
5 (20.0) |
| Small for gestational age |
| Yes |
2 |
1 (50.0) |
1 (50.0) |
0.25 |
| No |
24 |
20 (83.3) |
4 (16.7) |
| Seizure on-set |
| <3 days |
2 |
2 (100.0) |
0 (0.0) |
0.65 |
| 3-7 days |
21 |
17 (81.0) |
4 (19.0) |
| ≥ 7 days |
3 |
2 (66.7) |
1 (33.3) |
| Seizure type |
| Subtle |
3 |
2 (66.7) |
1 (33.3) |
0.054 |
| Focal/multifocal clonic |
20 |
18 (90.0) |
29 (10.0) |
| Tonic |
3 |
1 (33.3) |
2 (66.7) |
| Myoclonic |
0 |
0 (0.0) |
0 (0.0) |
| EEG findings |
| Normal/mildly abnormal |
20 |
18 (90.0) |
2 (10.0) |
0.029 |
| Moderate to severe abnormal |
6 |
3 (50.0) |
3 (50.0) |
| Follow-up EEG |
| Normal |
21 |
19 (90.5) |
2 (9.5) |
0.01 |
| Abnormal |
5 |
2 (40.0) |
3 (60.0) |
Table 1: Analysis of predictive factors on neurological outcomes.
Without specific perinatal complications and were relatively stable before seizure on-set. Two of these five infants required endotracheal intubation and mechanical ventilation owing to severe apneic episodes with uncontrolled seizures [9,10].
Similar clinical manifestations; they were delivered Laboratory test results
C-reactive protein levels were within the normal reference range in all patients (<0.5 mg/dL). Hypocalcemia (total calcium <8.0 mg/dL and ionized calcium 1.0 mmol/L) was detected in five infants at the time of seizure, but the seizures were not controlled by intravenous calcium administration. We performed serological viral studies including toxoplasma, rubella, cytomegalovirus, and herpes virus in all infants, but there was no positive result. Rotavirus antigens were detected in stool specimens from all 26 infants (100%). Additional rotavirus PCR testing of stool specimen was performed in 5 infants and they showed all positive results. Cerebro-spinal fluid (CSF) analysis was performed in 19 infants; pleocytosis was not observed (range 0-5 cells/mm3). The protein and glucose levels of CSF were all within the reference ranges (48-125 mg/dL and 47-132 mg/dL, respectively). For 19 infants, who were subjected to viral tests including herpes simplex virus, and enterovirus in CSF specimens, no viruses were detected [11].
EEG findings
EEG was performed within 24 hours, 48 hours, and 72 hours after seizure on-set in 8, 16, and two infants, respectively. Most infants (24/26, 92.3%) were evaluated with EEG within 48 hours after seizure. Moderate to severe abnormal EEG results were found in six infants (23.1%). Over two thirds of the infants (20/26, 76.9%) showed normal or relatively mildly abnormal EEG findings. Follow-up EEG was performed on 20 infants who showed abnormal findings in the initial EEG workup. EEG normalization was found in 15 infants within 3 months after discharge. The remaining five infants showed persistent abnormal findings on the EEG ranging from mildly delayed maturation to definite epilepsy. Severity of ictal background activities in EEG and normalization of follow-up EEG were associated with neurological outcomes on univariate analysis (Table 1).
Brain MRI findings
Brain MRIs were performed in 26 infants within 72 hours after seizure. All infants showed typical diffusion-restricted lesions in the symmetric cerebral white matter in DWIs (Figures 2 and 3), but four infants did not show any abnormal findings in white matter in T1-weighted images. The next most common involved site was the corpus callosum (22/26, 84.6%). Less commonly involved sites were the internal capsule, thalamus, anterior commissure, and external capsule, in order of frequency (69.2%, 65.4%, 26.9% and 23.1%, respectively).

Figure 2: Diffusion-weighted images of the one patient.
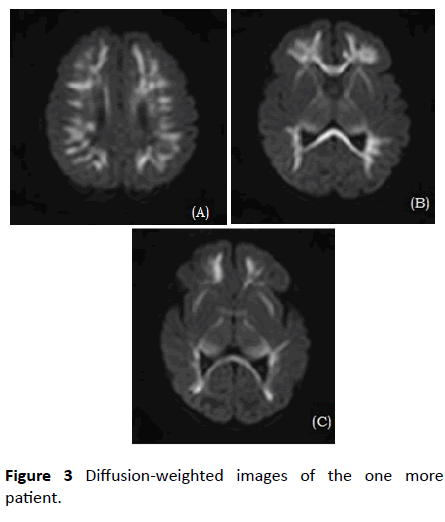
Figure 3: Diffusion-weighted images of the one more patient.
Mortality and neurodevelopmental outcomes
During the study period, no patients died. Favorable outcomes were observed in 21 infants (80.8%) and unfavorable outcomes in five (19.2%). The mean follow-up period was 18.19 ± 7.77 months (12-49 months).
One infant showed recurrent seizures and was diagnosed with infantile spasm at 9 months of age. Two infants were diagnosed with GDD, and they all showed motor and speech delay. Two infants were diagnosed with cerebral palsy. Follow - up brain MRI was performed in four infants among five with adverse neurological outcomes, two showed negative findings and two showed multiple patch signal changes with mild volume loss in cerebral white matter indicating leukomalacia change (Figure 4). Low birth weight and moderate to severe abnormal background EEG activities were associated with adverse neurologic outcomes in univariate analysis, but we could not determine a specific risk factor for adverse neurological outcomes on multivariate analysis.
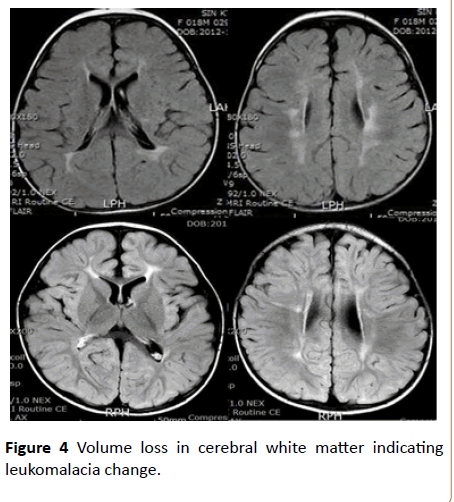
Figure 4: Volume loss in cerebral white matter indicating leukomalacia change.
Discussion
Rotavirus is a major causative pathogen of gastroenteritis in pediatric population, primarily in young infants and children. In addition, previous studies have indicated that rotavirus can also cause variable systemic extra-intestinal manifestations, including neurological symptoms, necrotizing enterocolitis in preterm infants, and several autoimmune diseases [11,12]. Especially, rotavirus infection has been linked to bradycardiaapnea episodes of neonates, childhood seizures, and clinically mild encephalitis/encephalopathy with a reversible splenial lesion [13,14], and thereby numerous reports describe the protective effects of rotavirus vaccination in reducing childhood hospitalization for seizures [15,16]. Neonates are vulnerable to seizures and rotavirus infection. However, rotavirus is usually underestimated as a cause or pathogen in neonates with seizures and it still does not constitute one of the elementary items for routine diagnostic work-ups for seizures in infants without gastroenteritis symptoms.
Recently, several small case series reported that rotavirus infection is also associated with cerebral white matter injury (WMI) in neonates, resulting in cystic periventricular leukomalacia [4,8-10,17,18]. Even if there are no signs of infection or gastroenteritis symptoms, rotavirus should be considered as a cause of neonatal seizures, especially in healthy full-term neonates with seizures around 5 days of life. Initial brain MRI showed typical diffusion restriction in periventricular WM, and leukomalatic change including cystic evolution was determined at follow-up MRI work-ups.
Our study did not perform rotavirus testing in the CSF. Furthermore, there was no pleocytosis in CSF examinations of 19 infants; other studies have also reported similar results that rotavirus was not usually detected in the CSF of infants with rotavirus-associated neurological complications [4,9]. In addition, the pattern of WMI seen in rotavirus infection is not specific. Other viruses, including enterovirus and human parechovirus also cause similar WM lesions. We evaluated and excluded herpesvirus and enterovirus in the CSF examination. In considering human parechovirus, clinical features are somewhat different: human parechovirus is usually manifested by sepsis-like illness including fever, skin rash and lethargy, and the on-set is more heterogeneous with a wide time range. Therefore, we excluded other viral causes of WMIs. The pathophysiology of central nervous system injury associated with rotavirus infection has not been fully-understood. Among several mechanisms that have been postulated, direct CNS infection by rotavirus is the most plausible explanation [19-21]. It has been demonstrated that rotavirus can infect neuronal cells directly. This is supported by several observational studies that rotavirus particles and RNA were detected in the CSF of infants with neurological symptoms [22,23]. Other indirect mechanisms have been suggested. Rotavirus non-structured protein 4 (NSP4) has been regarded as a cause of rotavirus-associated CNS complications [24]. It has been identified in dendritic processes [25] and has been shown to induce disruption of calcium homeostasis and release of nitric oxide metabolites in intestinal epithelial cells [26]. Additionally, it triggers secretion of pro-inflammatory cytokines from macrophage-like cell lines via Toll-like receptor 2 and also stimulates the release of peripheral inflammatory cytokines, such as interleukin-6 [27]. Although there is no obvious evidence that rotavirus caused the white matter injuries, it is certain that a significantly higher number of neonates presenting with seizures and distinct white matter lesions, observed in DWI, showed positive result in rotavirus tests compared to infants with seizure without characteristic DWI findings (100.0% vs. 8.8%). How rotavirus leads to WMIs remains unclear and further studies on this issue are needed.
In this present study, 20% of enrolled infants showed unfavorable neurodevelopmental outcomes. This is thought to be low compared to the incidence of long-term neurological sequelae after neonatal seizures caused by other etiologies including HIE, inherited metabolic disorder, or congenital developmental disorder of the brain [28-30]. However, we cannot be so optimistic considering that most had favorable outcomes in older infants and children with convulsions with mild rotavirus gastroenteritis [31]. In general, older infants and children with benign convulsion associated with rotaviral gastroenteritis are known to show normal or transient splenial lesions of the corpus callosum in brain MRI and they usually achieve normal development [32-35]. Therefore, neonatal seizure with WMIs accompanying rotavirus infection is a unique clinical entity apart from the benign convulsion with mild gastroenteritis seen in older ages. The prognosis is variable according to the age at infection, and may be because the immature, developing brain is more vulnerable to infectious insults.
This study has some limitations. First, we could not determine the mechanism of rotavirus induced white matter injury. However, we could not locate any other possible causes for the seizures except for rotavirus infection in enrolled patients through comprehensive work-ups. Second, this study was conducted retrospectively; consequently, diagnostic and therapeutic methods were not uniformly applied in all enrolled patients. Further, we could not determine the risk factors for neurological outcomes owing to limited statistical power due to the small sample size. Moreover, the follow-up period was too short to evaluate long-term neurologic outcomes and follow-up brain MRI was not performed in all patients. However, this study described clinical features and short-term neurological outcomes in neonates presenting with seizures and suggested the association of rotavirus infection and white matter injuries in these infants [34,36].
Conclusion
In conclusion, rotavirus should be considered in neonates presenting with seizures especially in neonates with a distinctive clinical course as described. Furthermore, pediatricians need to allow for possibility of WMIs in these infants. Although the available data do not sufficient for drawing conclusions on the association between brain lesions and rotavirus infection, the prognosis in such cases is not always benign, as reported by several studies. Therefore, close attention should be paid and follow-up for long-term neurological outcomes is required for infants with rotavirusinduced seizures resulting in white matter injury.
References
- Soni S, Anandjiwala S, Patel G, Rajani M (2008) Validation of different methods of preparation of Adhatoda vasica leaf juice by quantification of total alkaloids and vasicine. Indian J Pharm Sci 70: 36-42.
- Dash RP, Chauhan BF, Anandjiwala S, Nivsarkar M (2010) Comparative pharmacokinetics profile of Vasa Swaras with vasicine and vasicinone. Chromatographia 71: 609-15.
- Vyas T, Dash RP, Anandjiwala S, Nivsarkar M (2011) Formulation and pharmacokinetic evaluation of hard gelatin capsule encapsulating lyophilized Vasa Swaras for improved stability and oral bioavailability of vasicine. Fitoterapia 82: 446-453.
- Singh N, Chaudhary A (2011) A comparative review study of Sneha Kalpana (Paka) vis-a-vis liposome. Ayu 32: 103.
- Khan NA (2017) A conceptual study of ayurvedic management of optic atrophy. IAMJ 1: 6.
- Syam Chandran C, Nara A (2016) Ayurvedic adaptation to diabetic retinopathy. World J Pharm Med Res 2: 26-29.
- Senadheera T, Samarakoon SM (2017) A comparative clinical study on the efficacy of oral Sarasvata Ghrita and Sarasvata Ghrita Nasya in the management of Vataja Shirah Shoola (tension headache). "Salakya Sandipani", Department of Shalya Shalakya, Gampaha Wickramarachchi Ayurveda Institute, University of Kelaniya, Sri Lanka 50.
- Patil RR, Patil DB, Adnaik RS, Magdum CS (2011) Development and evaluation of a polyherbal formulation: Saraswat Ghrita. A Deccan J Nat Prod 2: 29-32.
- Mahajan NK, Singhai KA, Vadnere PG (2011) Investigation on anticataract activity of Triphala Ghrita. J Chem 8: 1438-1443.
- Soni RK, Srivastava G, Dhiman KS, Manjusha R, Mehta AJ (2015) Study on age related macular degeneration (dry type) in context to pitta vidagdha drishti and its Ayurvedic management. Int J Ayurveda Pharma Res 2.
- Rastogi S, Chawla S, Singh RP (2009) Ayurvedic management of unilateral loss of vision following a blunt injury to eye: A case report. Complementary health practice review 14: 84-92.
- CR Kim (2013) Neonatal rotavirus infection. Neonatal Med 20: 389-401.
- Takanashi J (2009) Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev 31: 521-528.
- Kato Z, Orii KE, Morimoto M, Sasai H, Funato M, et al. (2009) A transient lesion in the corpus callosum during rotavirus infection. Pediatr Neurol 41: 467-469.
- Pardo-Seco J, Cebey-López M, Martinón-Torres N, Salas A, Gómez-Rial J, et al. (2015) Impact of Rotavirus Vaccination on Childhood Hospitalization for Seizures. Pediatr Infect Dis J 34: 769-773.
- Park SH, Kim YO, Kim HK, Kim HS, Kim BY, et al. (2015) Incidence of benign convulsions with mild gastroenteritis after introduction of rotavirus vaccine. Brain Dev 37: 625-630.
- Yeom JS, Park CH (2016) White matter injury following rotavirus infection in neonates: new aspects to a forgotten entity, fifth day fits’? Korean J Pediatr 59: 285-291.
- Park JN, Park HA, Shin YH, Hwang JH (2017) Rotavirus-induced neonatal seizures with cerebral white matter abnormalities on magnetic resonace imaging: a case report. Neonatal Med 24: 45-48.
- Dura-Trave T, Yoldi-Petri ME, Gallinas-Victoriano F, Molins-castiella T (2011) Infantile convulsions with mild gastroenteritis: as retrospective study of 25 patients. Eur J Neurol 18: 273-278.
- Cusmai R, Jocic-Jakubi B, Cantonetti L, Japaridze N, Vigevano F (2010) Convulsions associated with gastroenteritis in the spectrum of benign focal epilepsies in infancy: 30 cases including four cases with ictal EEG recording. Epileptic Disord 12: 255-61.
- Nishimura S, Ushijima H, Nishimura S, Shiraishi H, Kanazawa C, et al. (1993) Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev 15: 457-9.
- Liu B, Fujita Y, Arakawa C, Kohira R, Fuchigami T, et al. (2009) Detection of rotavirus RNA and antigens in serum and cerebrospinal fluid samples from diarrheic children with seizures. Jpn J Infect Dis 62: 279-83.
- Fuchigami T, Goto K, Hasegawa M, Saito K, Kida T, et al. (2013) A 4-year-old girl with clinically mild encephalopathy with a reversible splenial lesion associated with rotavirus infection. J Infect Chemother 19: 149-53.
- Dickey M, Jamison L, Michaud L, Care M, Bernstein DI, et al. (2009) Rotavirus meningoencephalitis in a previously healthy child and a review of the literature. Pediatr Infect Dis J 28: 318-321.
- Di Fazio MP, Braun L, Freedman S, Hickey P (2007) Rotavirus-induced seizures in childhood. J Child Neurol 22: 1367-1370.
- Shiihara T, Watanabe M, Honma A, Kato M, Morita Y, et al. (2007) Rotavirus associated acute encephalitis/encephalopathy and concurrent cerebellitis: report of two cases. Brain Dev 29: 670-673.
- Weclewicz K, Svensson L, Kristensson K (1998) Targeting of endoplasmic reticulum-associated proteins to axons and dendrites in rotavirus-infected neurons. Brain Res Bull 46: 353-360.
- Rodr J, Banasaz M, Istrate C, Buesa J, Lundgren O, et al. (2006) Role of nitric oxide during rotavirus infection. J Med Virol 78: 979-985.
- Ge Y, Mansell A, Ussher JE, Brooks AE, Manning K, et al. (2013) Rotavirus NSP4 triggers secretion of proinflammatory cytokines from macrophages via Tolllike receptor 2. J Virol 87: 11160-7.
- et al. (2012) Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol 47: 186-92.
- Garfinkle J, Shevell MI (2011) Prognostic factors and development of scoring system for outcome of neonatal seizures in term infants. Eur J Pediatr Neurol 15: 222-229.
- Garfinkle J, Shevell MI (2011) Cerebral palsy, developmental delay, and epilepsy after neonatal seizures. Pediatr Neurol 44: 88-96.
- Verrotti A, Tocco AM, Coppola GG, Altobelli E, Chiarelli F (2009) Afebrile benign convulsions with mild gatroenteritis: a new entity? Acta Neurol Scand 12: 73-79.
- Karampatsas K, Spyridou C, Morrison IR, Tong CY, Prendergast AJ (2015) Rotavirus-associated mild encephalopathy with a reversible splenial lesion (MERS)-case report and review of the literature. BMC Infect Dis 15: 446.
- Fuchigami T, Goto K, Hasegawa M, Saito K, Kida T, et al. (2013) A 4-year-old girl with clinically mild encephalopathy with a reversible splenial lesion associated with rotavirus infection. J Infect Chemother 19: 149-153.
- Natsume J, Naiki M, Yokotsuka T, Sofue A, Ikuta T, et al. (2007) Transient splenial lesions in children with ‘‘benign convulsions with gastroenteritis.’’ Brain Dev 29: 519-521.




