Research Article - (2021) Volume 6, Issue 1
KR.Ranga Reddy1, Dr. K. V. Sastry1, Dr. V. Uma Maheshwara Rao1
1M.Pharm, Ph.D
*Corresponding author: Ranga Reddy M.Pharm, India, Tel: +91 897656565555; E-mail: rangareddy.kr1@gmail.com
Received date: May 27, 2020; Accepted date: August 12, 2021; Published date: August 22, 2021
Citation:Ranga Reddy (2021) Nanosponges in Drug Delivery. Trends Green Chem. Vol.7 No.1.
Nanosponges are porous polymeric delivery systems that are tiny spherical particles which contain nanometer sized cavities with high porous surface, in which a variety of drugs can be encapsulated and improve the solubility of poorly water soluble drugs. These particles have capacity of carrying both hydrophilic and lipophilic drugs[1]. Theyforma suspension when dispersed in water, thereby forming a matrix-like structure in aqueous media, allowing a free transfer of entrapped drug molecules and prolong the drug release. These have aproperty of forming a sponge-like structure and have high capacity to entrap small molecules in its matrix.The total solubility effect exerted by Nanosponges may be because of entrapment in the matrix as well as the formation of inclusion complex with drug.Therefore, Nanosponges can form both inclusion and non-inclusion complexes. The ability of these to form non-inclusion complexes with drug molecules may be because they are consisting of more than one cyclodextrin units closely associated with each other[2].
INTRODUCTION
Nanosponges are porous polymeric delivery systems that are tiny spherical particles which contain nanometer sized cavities with high porous surface, in which a variety of drugs can be encapsulated and improve the solubility of poorly water soluble drugs. These particles have capacity of carrying both hydrophilic and lipophilic drugs[1]. Theyforma suspension when dispersed in water, thereby forming a matrix-like structure in aqueous media, allowing a free transfer of entrapped drug molecules and prolong the drug release. These have aproperty of forming a sponge-like structure and have high capacity to entrap small molecules in its matrix.The total solubility effect exerted by Nanosponges may be because of entrapment in the matrix as well as the formation of inclusion complex with drug.Therefore, Nanosponges can form both inclusion and non-inclusion complexes. The ability of these to form non-inclusion complexes with drug molecules may be because they are consisting of more than one cyclodextrin units closely associated with each other[2].
Polyester nanosponges contain a backboneof naturally degradable polyester. The long length polyester strands mixed with cross linkers in solution that have affinity for polyester. They cross link with segments of the polyester to form a spherical shape that has many cavities in which drugs can be incorporated. The polyester is biodegradable which breaks up in the body from which drug can be released.These sponges circulate in the body until they recognize specific target site and stick to the surface, then release the drug in a controlled and prolonged manner.Because the drug can be released at the specific target site instead of circulating throughout the body it will be more effective for a particular given dose. Drug loaded nanosponges can be used for treatment of many diseases and recent studies show this technology is five times more effective in delivering the drugs for breast cancer than conventional systems. Another important character of these polyester nanosponges is their aqueous solubility; this allows the use of these systems effectively for drugs with poor aqueous solubility[3].
Nanosponges are encapsulating type of nanoparticles which encapsulates the drugs within its core. Based on the method of association with drugs, the nanoparticles can be classified into encapsulating nanoparticles, complexing nanoparticles and conjugating nanoparticles.Encapsulating nanoparticlesinclude nanosponges and nanocapsules. Alginate nanoparticles are sponge like nanoparticles which contain many cavities that carry drug molecules. Nanocapsules such as poly (isobutyl cyanoacrylate) are also encapsulating type nanoparticles. They can entrap the drug molecules in their aqueous core. In complexing nanoparticles type the molecules are attracted by electrostatic charges but in conjugating nanoparticles which links to drugs through covalent bonds[4].
Nanosponges are a novel class of nanoparticles which are porous, nontoxic, and stable at high temperatures up to 300°C.They are able to capture, transport and selectively release a huge variety of substances because of their 3D structure containing cavities which are in nanometer size.Cyclodextrin based Nanosponges are stable in water and they slowly undergo hydrolysis leading to the parent cyclodextrins[5].
ADVANTAGES
DISADVANTAGES
PREPARATION OF NANOSPONGES
Synthesis of nanosponges
Nanosponges can be synthesized by using three methods. They are
Emulsion solvent diffusion method: Nanosponges are prepared by using different proportion of ethyl cellulose and polyvinyl alcohol. The dispersed phase which contains ethyl cellulose dissolved in 20ml dichloromethane and slowly added to adefinite amount of polyvinyl alcohol in 150ml of aqueous continuous phase. The reactionmixture was stirred at 1000rpm for 2 hrs. The nanosponges formed were collected by filtration and dried in oven at 40°C for 24 hrs.The dried nanosponges were stored in vacuum desiccators for removal of residual solvent.The mean particle size of nanosponges can be greatly influenced by drug: polymer ratio. The low concentration of polymer selected enhances the diffusion of dichloromethane into aqueous phase thus providing less time for the droplet formation and hence decreases the particle size[1].
Ultrasound assisted synthesis: In this method Nanosponges can be obtained by reacting polymers with cross linking agents in the absence of any solvent and under sonication. Mix the polymer with cross linking agent in a particular molar ratioin a flask. Place the flask in an ultrasound bath filled with water and heat it to 90°C. Sonicate the mixture for an about 5hours. Then allow the mixture to cool and break the product. Wash the product with distilled water to remove the unreacted polymer and purify the product by prolonged Soxhlet extraction with ethanol. Dry the obtained product under vacuum and store it at 25°C. The main advantage of this method is the nanosponges will be spherical and uniform in size[6].
Solvent method: The polymer either polyester or β-cyclodextrin was mixed with a suitable solvent that is in a polar aprotic solvent like Dimethylformamide (DMA), Dimethylsulfoxide (DMSO). Then add this mixture to the excess quantity of cross linking agent. In general 4:16 molar ratio of cross linking agent and polymer was used to achieve better cross linking between the polymer and cross linking agent. The reaction was carried out at a temperature ranging from 100C to reflux temperature of the solvent for about 1 to 48h. The cross linking agents mainly used are carbonyl compounds e.g. Dimethyl carbonate and Carbonyldiimidazole. After completion of the reaction, solution was allowed to cool at room temperature, and then the obtained product was added to large excess of bidistilled water and recovers the product by vacuum filtration. Then the product was purified by prolonged Soxhlet extraction with ethanol. Dry the product under vacuum and grind in a mechanical mill to obtain a homogeneous powder[7].
Table1: Polymers used in synthesis of nanosponges.
| S.NO | POLYMERS | EXAMPLES |
|---|---|---|
| 1. | Cyclodextrin derivatives | β-CyclodextrinMethyl β-cyclodextrin2-Hydroxy Propyl β-CyclodextrinAlkyloxycarbonyl Cyclodextrins |
| 2. | copolymers | Poly(valerolactoneallylvalerolactone)Poly(valerolactone-allylvalerolactone-oxepanedione) |
| 3. | Cellulose derivatives | Ethyl cellulose |
| 4. | Hyper crosslinked polymers | polystyrenes |
The cross linking agents play an important role in the synthesis of nanosponges. The synthesized nanosponges have a specific size and release the drugs in a controlled manner which depends on the proportion of cross linking agent to polymer ratio.
Table2: Cross linking agents used in synthesis of nanosponges.
| S.NO | CROSSLINKERS | EXAMPLES |
|---|---|---|
| 1. | Carbonates | Diphenyl carbonateDiaryl carbonate |
| 2. | Cyanates | Diisocyanate |
| 3. | Aldehydes | Glutaraldehyde |
| 4. | Carboxylic acids | Carboxylic acid dianhydride2,2-bis(acrylamido) Acetic acid |
| 5. | Imidazoles | Carbonyl diimidazole |
Loading of drugs into Nanosponges
The prepared Nanosponges were suspended in water and sonicated to avoid the presence of aggregates then the suspension was centrifuged to obtain a colloidal fraction. Excess amount of drug was added to aqueous suspension of Nanosponge,and maintain the suspension underconstant stirring for 24h required for complexation. After 24h, the suspension was centrifuged at 2000rpm for 10min to separate the uncomplexed drug as a residue below the colloidal supernant. Then obtain the solid crystals of Nanosponges by solvent evaporation or by freeze drying.It was stored in vacuum desiccators at room temperature. Crystal structure of nanosponge plays a very important role in complexation with drug. Paracrystalline nanosponges show different loading capacities when compared to crystalline nanosponges. The drug loading is greater in crystalline nanosponges than paracrystalline one[8].SSS
DRUGS USED IN NANOSPONGE DRUG DELIVERY
Econazole nitrate
Econazole is an imidazole antifungal used topically to relieve the symptoms of superficial candidiasis, dermatophytosis, pityriasis and skin infections. Econazole nitrate is commercially available as 1% cream, 1% ointment, 150 mg vaginal tablet, lotion, powder and solution. These delivery systems require a highconcentration of active agents to be incorporated for effective therapy because of their low efficacy as delivery systems. There is a need of delivery system to maximize the time period that an active ingredient ispresent on the skin, while minimizing its penetration in to the body. Econazole nitrate is slowly metabolized by the skin thereforecontrolling the release of drug will improve the efficacy of formulation and decrease the frequency of application. Thus nanosponges-based topical delivery systems that will overcome the limitations associated with conventional formulations and reduce side-effects like burning, stinging sensations, contact dermatitis.Econazole nitrate nanosponges were prepared by emulsion solvent diffusion method and these nanosponges were loaded in hydrogel as a local depot for sustained drug release.Econazole nitrate-loaded nanosponges show prolonged drugrelease and therefore produce some benefitssuch as reduction in total dose, frequency of administrationand dose-related systemic side-effects[1].
Tamoxifen
Tamoxifen is a weak base with low aqueous solubility and have a melting point of 100°C. Commercially,Tamoxifen base is converted to the citrate salt to increase the aqueous solubility and efficacy. However, the increase in its solubility via salt formation is limited due to the higher meltingpoint of Tamoxifen citrate (146°C) which inhibits dissolution rate.Tamoxifen is given to patients for long periods of time and is used for the treatment of breast cancer in pre- and postmenopausal women. It has major side effects like endometrial carcinoma, liver cancer, venous thrombosis, pulmonary emboli and ocular effects which are dose and concentrationdependent. This will limit the Tamoxifen application. Hence there is need to develop prolongrelease formulation of Tamoxifen which can minimize the side effects. Tamoxifen is loaded into nanosponges which increase solubility and release the Tamoxifen in a controlled manner[3].
Camptothecin
Camptothecin is a plant alkaloid which is a potent anticanceragent acting through the inhibition of topoisomerase I during the S-phase of the cell cycle. Camptothecin and its derivatives have showna wide spectrum of anticancer activity against human malignanciesincluding human lung, prostate, breast, colon, stomach, ovariancarcinomas, melanoma, lymphomas and sarcomas.Despite of this high activity, it has a limited therapeutic utility. This is due to its poor aqueous solubility, seriousside effects and opening of the lactone ring at physiologicalpH to yield the carboxylate form which is inactive. In addition,the ring-opening results in charged drug species exhibitinglimited permeability through the lipid bilayer of a low dielectricconstant thereby alter the molecular diffusivity.Extensive researches have been carried out to develop deliverysystems for the insoluble lactone form of Camptothecin and its derivatives. These include entrapment into liposomes, microspheres, nanoparticles, complexation with lipids or cyclodextrins and preparation of macromolecular prodrugs. A new formulation has been developed for Camptothecin which consist of the encapsulation in nanosponges for prolongingthe shelf life and release of the drug. The Nanosponges may solubilize Camptothecin by complexation and may protect the lactonering from opening due to its high inclusion abilities thereby increasing the drug stability[8].
Paclitaxel
Paclitaxel is an anti-neoplastic agent derived from the bark of the Pacific yew tree (Taxus brevifolia). Paclitaxel is primarily metabolized in the liver and undergoes biliary excretion. Orally administered paclitaxel has a therapeutic problem because of low bioavailability due to low solubility, dissolution,high first-pass metabolism that occurs in the liver and transport processes due to P-glycoprotein in the intestinal wall. The oral bioavailability of paclitaxel can be improved by incorporating it into nanosponges a novel drug delivery system. Administration of paclitaxel-loaded cyclodextrin nanosponges forms amatrix-like structure in gastrointestinal fluids, therebysolubilizing the water-insoluble paclitaxel byforming a nanosuspension. At the
same dose, the AUC of 20 mg of paclitaxel loaded nanosponges (equivalent to 10 mg paclitaxel) is greater thanAUC of 10 mg of paclitaxel. Themean absolute bioavailability of paclitaxel loaded nanosponges was 2.5 fold higherthan paclitaxel[9].
Resveratrol
Resveratrol is a polyphenolic phytoalexin derivative and used for treatment of many diseases like inflammation, cardiovascular diseases, dermatitis, gonorrhea,fever and hyperlipidemia. Due to hydrophobicity, dissolution is a rate limiting step for in vivo absorption, which constitutes problem for oral bioavailability. To overcome this problem, Resveratrol is loaded into nanosponges which can improve the solubility and dissolution rate[10].
FACTORS INFLUENCE NANOSPONGE FORMATION
Type of polymer
Type of polymer, type of cross-linking agent and ratio of polymer to cross-linker influence the nanosponge formation and these will also influence the rate of release of drug from nanosponges.The mean particle size of nanosponges can be greatly influenced by drug: polymer ratio.
Type of drug
Temperature
The temperature changes can affect Drug- Nanosponge complex formation.In general increasing in the temperature decreases the magnitude of apparent stability constant of drug-nanosponge complex. This may be due to the reduction of drug-nanosponge interaction forces such as VanderWaal forces and hydrophobic forces with rise of temperature[12].
Method of preparation
The method of loading the drug into the nanosponge can affect Drug-Nanosponge complexation. However, the effectiveness of a method depends on the nature of the drug; polymer and freeze drying. Freeze drying wasfound to be most effective for drug complexation[12].
Degree of substitution
The complexation ability of the nanosponges can be greatly affected by type, number and position of substituent on the parent molecule[12].
CLASSIFICATION OF NANOSPONGES
CYCLODEXTRIN BASED NANOSPONGES
Beta-cyclodextrin is cyclic oligosaccharides consisting of seven glucopyranose units which form inclusion complex with guest molecules due to special property-hydrophobic internal cavity and hydrophilic external surface. They have capability to include compounds whose geometry and polarity are compatible with the cavity of cyclodextrin. Most useful cyclodextrins have low water solubility and is toxic when injected intravenously. Somecyclodextrin derivatives are well tolerated parenterally like 2-hydroxyl propyl beta-cyclodextrin[13]. The usage of individualCyclodextrins and its derivatives are limited because they dissociate from the drug on dilution due to presence of individual Cyclodextrins or individual cyclodextrin derivatives. This drawback of cyclodextrin can be overcome by synthesizingdimers or timers that can improveformation of inclusioncomplexes with the guest molecules. Cross linking agents are used to cross link the individual cyclodextrin to form a spherical shaped structure which contain nanosized cavities. The best network is generated using epichloridrine as cross-linking agent. TheseCyclodextrins have been used for several purposes,including column packing for inclusion chromatography, elimination of bitter components from grapefruit juice, for copper analysis, and cobalt determination in foods.The primary purpose of synthesizing Nanosponges was enhancement in solubilization efficiency over the conventional cyclodextrins.
Cyclodextrin based nanosponges can be obtained by cross-linking different types ofCyclodextrins with a carbonyl (or) a dicarboxylate compound as cross-linking agent. Glutaraldehyde is also giving a cross-linked Cyclodextrins. Other cross linking agents which can form these nanosponges includes diisocianates, diary carbonates and carbonyl diimidazoles, carboxylic acid dianhydrides and 2, 2-bis (acrylamido) acetic acid. Nanosponges are prepared by hyper-cross-linked cyclodextrins to form 3-dimensional networks- a roughly spherical structure, which contain channels and pores inside. The surface charge density, porosity and pore sizes of sponges can be controlled by attaching with different molecules.They are solid particles with a spherical morphologythat have a very high solubilizing capacity for poorly soluble molecules.They canform bothinclusion and non-inclusion complexes with different drugs.The ratio of cyclodextrin and cross-linker can be varied during their preparation to improve the drug loading and to control the release of drug from nanosponge. The solubility and entrapment efficiency is dependent on the ratio of drug and crosslinking agent.
Cyclodextrin based nanosponges can be synthesized by adding equimolar volume of beta-cyclodextrin and NaOH to a 500ml round bottom flask containing 200ml distilled water and stirred at 700C till a transparent solution was formed. Then E-51(Epoxy resin with epoxy value=0.51) was dissolved in 100ml ethanol and this solution was added to the bet-cyclodextrin solution in drowise. This mixture was stirred for 24h at 700C to obtain nanosponges[14].
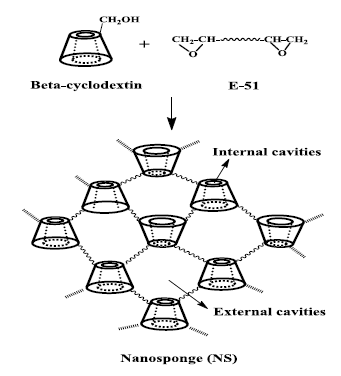
Fig1: Reaction between beta-cyclodextrin and Epoxy resin leading to formation of nanosponge.
Nanosponges are synthesized in neutral or acidic forms, depending on thecross linking agent used. They are solid nanoparticles and can be prepared in crystalline form with spherical shape using an ultrasound-assisted preparation method. The capacity of the nanosponges to incorporate molecules within their structurewas evaluated using drugs with different structures and solubility.Solubility is more in case of Nanosponge formulation containing higher cross linker because more entrapment in matrix as well as formation of inclusion complex with drug.
CHARACTERIZATION OF NANOSPONGES
Nanosponges can be characterized by using the following methods or techniques.
Particle size and polydispersity
The particle size of nanosponges can be determined by Dynamic light scattering using 90 plus particle sizer. The measurements were made at a fixed angle of900 for all samples at a temperature of 250C. The dispersions were diluted withbidistilled water for every measurement. Each sample was analyzed in triplicate.The mean hydrodynamic diameter(Dh) and polydispersity index, of the particles werecalculated in intensity using the cumulant analysis afteraveraging the three measurements. Dh was derived fromthe measured translational diffusion coefficient of particlesmoving under Brownian motion[3].
Zeta potential
It is a measure of the level of the surface charge and serves as an indicator of the relative magnitude of the repulsion force between colloidal particles in aqueous suspension.A highzetapotentialwill prevent particle-particle agglomeration. If the zeta potential is greater than 30mV, then the dispersion is stable. It can be measured by using additional electrode in particle size equipment. The samples were diluted with 0.1mM KCl and placed in electrophoretic cell, where electric field of 15V/cm was applied[8].
Microscopic studies
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) can be used to study the microscopic aspects of the drug, nanosponges and the product (drug/nanosponge complex). The difference in crystallization state of the raw materials and the product seen under electron microscope indicates the formation of the inclusion complexes.TEM studies of Camptothecin loaded nanosponges showed that the regular spherical shape of nanosponges is unaffected even after drug encapsulation. The paracrystalline nanosponges have larger average diameter than thecrystalline ones. TEM measurements revealed mean particle sizeof about 400 nm and 900 nm for plain crystalline and paracrystalline nanosponges respectively and a size increase for the drugloaded crystalline nanosponge[8].
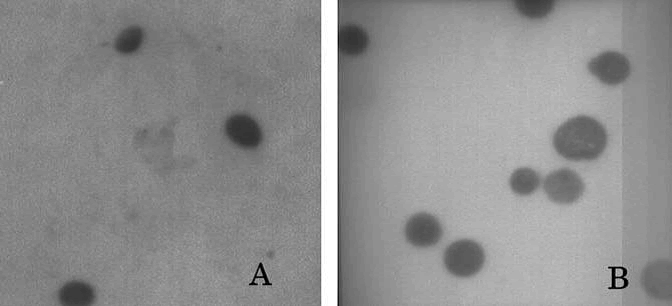
Fig 2: TEM of (A) blank nanosponges and (B) Camptothecin loaded nanosponges.
Solubilization efficiency
The solubilization efficiency of Nanosponges was estimated for comparing the solubilization enhancement capacity of nanosponges with their monomer i.e. β-cyclodextrin. The excessquantity of drug was suspended with fixed quantity of Nanosponges in distilled water. The same amount of drug was also suspended with fixed quantity of β-CD. The vials were placed on a mechanical shaker at ambient temperature for 24h. After this, un-solubilized drug was removed by filtering the suspension and filtrate was collected. The drug retained in nanosponges was extracted with ethanol and analyzed.S.Torne et al showed that the 1:8 (β-CD: Cross linker) nanosponge formulation of Tamoxifen show more solubilization efficiency compared to their monomer i.e.β-cyclodextrin[3].
Entrapment efficiency
For encapsulation efficiency, drug loaded nanosponges were separated from free drug. The drug loaded Nanosponges was diluted with water, centrifuged for 15 min at 10,000 rpm, and the supernant was removed.Sediment was taken out, dried at vacuum. Methanol was added and the free drug present in it was measured using suitable analytical method. The drug encapsulation efficiency was calculated by the following formula[9]:
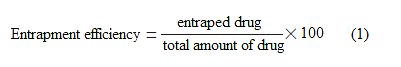
In vitro release studies
The in vitro release was carried out using multi-compartmentrotating cells with a dialysis membrane. The donor phase consisted of nanosponge formulation containinga fixed amount of drug.The multi-compartment rotating cell was rotated with 25rpm.The samples were withdrawn from receiving phase and replacedwith fresh medium after fixed time intervals; these are diluted and analyzed by using the HPLC.In vitrorelease of Tamoxifen from nanosponge complexes showed rapid and complete release. The difference in the release pattern from Nanosponges having high and low ratio of crosslinking agent shows that the release can be modulated by increasing or decreasing the crosslinking between the cyclodextrin molecules. This indicates that Nanosponges not only entrap higher amount of drug but also slow the release of drug from the matrix. The release of drug from Nanosponge can modulate by using different ratios of β-CD and cross linker [3, 8].
Thermal analytical methods
Thermo analytical methods identify whether the drug substance undergoes any change before the thermal degradation of the nanosponge. The change of drug substance may be due to melting, evaporation, decomposition,oxidation or polymorphic transition. The change of drug substance indicates the complex formation. The thermograms of DTA and DSC can be observed for broadening, shifting and appearance or disappearance of certain peaks. The changes in weight loss can also provide an indication of formation of complex[11].
E.g.The DSC thermogram of Tamoxifen showed that Tamoxifen has a melting point ofaround 100°C, showing endotherm at 99°C. But in the complex, this endotherm is suppressed, indicating the partial protection of Tamoxifen due to the encapsulation in Nanosponges[3].
X-ray diffractiometry
Powder X-ray diffractiometry can be used to detect inclusion complexation in the solid state. When the drug molecule is liquid since liquid have no diffraction pattern of their own, then the diffraction pattern of a newly formed substance clearly differs from that of uncomplexed nanosponge. This difference of diffraction patternindicates the complex formation. When the drug compound is a solid substance, a comparison has to be made between the diffractogram of the assumed complex and that of the mechanical mixture of the drug and polymer molecules.
A diffraction pattern of a physical mixture is often the sum of those of each component, while the diffraction pattern of complexes are apparently different from each constituent and lead to a newsolid phase with different diffractograms. Diffraction peaks for a mixture of compounds are useful in determining the chemical decomposition and complex formation.The complex formation of drug with nanosponges alters the crystalline nature of the drug. The complex formation leads to the sharpening of the existing peaks, appearance of a few new peaks and shifting of certain peaks.Single crystal X-ray structure analysis used to determine inclusion structure and mode of interaction between the host and guest molecules[11].
E.g. The intensity of Tamoxifen loaded Nanosponges is lower than that of plain drug. The crystalline peaks of plain drug was absent in Tamoxifen loaded Nanosponge formulation which indicates theencapsulation of Tamoxifen in Nanosponges. The encapsulateddrug may be in amorphous or solid state solubilizedform or in disordered crystalline phase within polymericmatrix[3].
Infra-red spectroscopy
Infra-Red spectroscopy is used to estimate the interaction between nanosponges and the drug molecules in the solid state.The IR bands of nanosponges change only slightly upon complex formation. If the fraction of guest molecules encapsulated in complex is less than 25%, the bands of it are masked by the bands of nanosponges. This technique is not suitable to detect the inclusion complex formation and is less used. The application of the Infra-red spectroscopy is limited to the drugs having some characteristic bands, such as carbonyl or sulfonyl groups.Infrared spectral studies give information regarding the involvement of hydrogen in various functional groups. Shift in the absorbance bands to the lower frequency, increases the intensity and widening of the band caused by stretching vibration of the group involved in the formation of the hydrogen bonds[15].
E.g. Cyclodextrin based nanosponges obtained by reaction of beta-cyclodextrin with Diphenyl carbonate. The presence of carbonate bond which has peak at around 1700-1750 cm-1 indicates formation of nanosponges.
The shift in IR spectrum of Resveratrol after complexation indicates formation of complex. Thereis intense peak due to O-H stretching typical to the carbohydratesin the spectrum of Nanosponges which is shifted to3,273 cm-1 in the complex, indicating there is interactionof OH groups of Nanosponge withResveratrol[10].
Haemolytic activity of Nanosponges
Nanosponges were incubatedat 37 0C for 90 min with 1 ml of diluted blood. Freshly preparedPBS (pH 7.4) was used for all dilutions.After incubation, blood containing suspensions were centrifugedat 2000 rpm for 10 min to separate plasma. The amount ofhaemoglobin released due to haemolysis was measured spectrophotometrically at 543 nm. The haemolyticactivity was calculated with reference to blank and complete haemolyzedsamples (induced by addition of ammonium sulfate 20%w/v). Optical microscopy was also used to see if there were anyabnormalities in the blood cells after incubation. The observationswere made with reference to the blank diluted blood[8].
In vitro cytotoxicity studies
In vitro cytotoxicity studies of nanosponges are carried on MCF-7 cell lines. MCF-7cells were collected and maintained in Dulbecco’s Modified Eagle’sMedium supplemented with 10% Fetal Calf Serum at37°C in a humidified incubator containing 5% CO2.Confluent cell monolayers were trypsinized and cellsin exponentially growing phase were used in cytotoxicityexperiments. The cytotoxicity of drug loaded nanosponges and drug against MCF-7 cells was assessed using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay. MCF-7 cells in culture medium were seeded in 96-wellplates and incubated for adherence at 37°C in 5% CO2. 20 μL of drug solution or a formulation was added to the wells. After 24 h of incubation, 20 μL MTT solution(5 mg/mL) was added to each well and the plates wereincubated for further 2 h. The solution in each well containingmedia, unbound MTT and dead cells was removed and 100 μL of DMSO was added to each well. Theplates were then shaken and the optical density was read at wavelength of 570 nm.Cells incubated in culture medium alone served as a controlfor cell viability (untreated wells)[3].
APPLICATIONS OF NANOSPONGES
Nanosponges in drug delivery[7]
Table3: Examples of nanosponges.
| S.NO | DRUG | INDICATION | NANOSPONGEVEHICLE |
|---|---|---|---|
| 1. | Itracanazole | Fungal infection | β-Cyclodextrin & copolyvidonum |
| 2. | Tamoxifen | Breast cancer | β-Cyclodextrin |
| 3. | Paclitaxel | Cancer | β-cyclodextrin |
| 4. | Econazole nitrate | Fungal infection | Ethyl cellulosePolyvinyl alcohol |
| 5. | Camptothecin | Cancer | β-Cyclodextrin |
| 6. | Temozolamide | Brain tumors | Poly(valerolactoneallylvalerolactone)poly(valerolactoneallylvalerolactone–oxepanedione) |
| 7. | Dexamethasone | Brain tumors | β-Cyclodextrin |
| 8. | Antisenseoligonucleotides | Cancer therapyViral infections | Sodium alginatePoly L-lysine |
| 9. | Resveratrol | Inflammation,Cardiovasculardiseases, Dermatitis,and Gonorrhea | β-Cyclodextrin |
Nanosponges in delivery of proteins, enzymes, vaccines and antibodies[16].
Proteins, peptides, enzymes used in the biomedical and therapeutic field. Photolytic enzymes can be used to treat cancer or type I mucopolysaccharidosis, while DNA and oligonucleotides are used in gene therapy.
The problems and limitations of administration of protein and peptide molecules include
Characteristics possessed by these proteins and peptides.
Large molecular size
Hydrophilic nature
Degree of ionization
High surface charge
Chemical and enzymatic instability and
Low permeability through mucous membranes
absorptionenhancers, which may lead to toxic problems.
A number of systems for carrying enzymes and proteins have been developed, such as nano and micro particles, liposomes and hydrogels.These will protect the proteins from breakdown, modify their pharmacokinetics and improve their stability in vivo.
Cyclodextrin based nanosponges are suitable carriers for proteins, enzymes, antibodies and macromolecules.Proteins and other macromolecules can be carried by adsorbing or encapsulating them in cyclodextrin nanosponges.
Bovine serum albumin is unstable in solution; they are stored in lyophilized state. However proteins can reversibly denatured on lyophilization and change conformation which is different from native structure. Major drawback in protein formulation and development is to maintain its native structure during processing and long term storage. Proteins like Bovine serum albumin are encapsulated in swellable cyclodextrin based poly (amidoamine) nanosponges to increase the stability of proteins.
Nanosponges in oxygen delivery system.
Gases play an important role in medicine, either for diagnostic ortreatment purposes.It is difficult to deliver oxygen inappropriate form and dosage in clinical practice.The deficiencyof adequate oxygen supply (hypoxia), leads to various pathologies from inflammation to cancer.
Cyclodextrin nanosponges are biocompatible nanoporousnanoparticles, obtained by the cross-linking of cyclodextrins, have capacity of encapsulatingactive molecules due to the presence of cyclodextrin cavities and cross-linker network.Cyclodextrin based nanosponges can act as an effective carrier for gases like oxygen and carbon dioxide. They form inclusion complex with gases like 1-methylcyclopropene, oxygen and carbon dioxide. The complexation of nanosponges with oxygen or carbon dioxide could beuseful for many biomedical applications. The oxygen-filled nanosponges could supply oxygen to the hypoxic tissues which are present in various diseases and these tissues require high amounts oxygen for carryout various physiological processes.Therefore Nanosponges were used for oxygen deliverysystems for topical applications[17].
CONCLUSION
Nanosponges improve the solubility of poorly soluble drugs and protect the active moieties from physicochemical degradation.These have ability to encapsulate either lipophilic or hydrophilic drugs.They can form both inclusion and non-inclusion complexes.The ratio of cyclodextrin and cross-linker can be varied to improve the drug loading and to control the release of drug from nanosponge.By controlling the ratio of polymer to the cross-linker the particle size and release rate can be modulated.Polyester nanosponges deliver the drugs to the target site three to five times more effectively than direct injection.These sponges circulate in the body until they recognize specific target site and stick to the surface, then release the drug in a controlled and prolonged manner. The tiny shape of nanosponges enables the pulmonary and venous delivery of nanosponges. Incorporation of nanosponges into hydrogel show better release and treatment of skin infections