Review Article - (2023) Volume 7, Issue 3
Nanoparticles Reactions in Cervical Cancer: Challenge and Hope
V Anusha Devi1* and
Kalaiselvi V2
1Department of Physics, Nandha Arts and Science College, Tamilnadu, India
2Department of Physics, Navarasam Arts and Science College for Women, Tamilnadu, India
*Correspondence:
V Anusha Devi, Department of Physics, Nandha Arts and Science College, Tamilnadu,
India,
Email:
Received: 03-Dec-2022, Manuscript No. IPNNR-22-15229;
Editor assigned: 06-Dec-2022, Pre QC No. IPNNR-22-15229 (PQ);
Reviewed: 20-Dec-2022, QC No. IPNNR-22-15229;
Revised: 20-Sep-2023, Manuscript No. IPNNR-22-15229 (R);
Published:
27-Sep-2023, DOI: 10.12769/IPNNR.23.7.022
Abstract
In this paper, a co-precipitation and thermal breakdown approach was used to create a CuO/SnO nanocomposite using CuO, NiO, and SnO as precursors. Sol-gel synthesis was used to create Nickel Oxide (NiO) nano-particles. The XRD (X-Ray Diffraction), SEM (Scanning Electron Microscopy), and FTIR (Fourier Transform Infrared Analysis) were used to analyze the nanocomposites as-prepared; CuO and SnO have wurtzite crystal structures that are cubic and hexagonal, respectively, as seen by the XRD diffractogram of a CuO/SnO nano-composite. The existence of CuO and SnO was verified by FTIR bands in the CuO/SnO nano-composite. The concept of CuO nano-particles mixed with Hibiscus flower reacted as anti-cancer property.
Keywords
Nano-composite; CuO; SnO; NiO; FTIR; XRD; Hibiscus flower; Anti-cancer study
Introduction
Two of the major drivers of pollution and energy consumption
are population growth and rapid urban/industrialization. By
discharging polluted and coloured effluent into the water
supply, synthetic organics used in industries like medicines,
textiles, cosmetics, paper, and plastics have seriously harmed
the environment. They degrade water quality, impede light
penetration, and reduce photosynthetic responses.
Furthermore, certain colours are both toxic and carcinogenic.
The aforementioned issues have been dealt with using a
variety of therapeutic techniques. In treatment procedures
such as adsorption, chemical precipitation, and coagulation
are inefficient and produce secondary pollutants such as
hazardous gases and slurry, which require extra purification.
As a result, innovative technology based methods for
pollution removal have been proposed.
Advanced Oxidation Processes (AOPs) have received a lot of
interest as a replacement for existing treatment approaches
for converting hazardous organic pollutants into innocuous
chemicals. AOPs have benefits including the ability to function
at normal temperatures and pressures and convert
environmental contaminants into sustainable products. The
energy levels of these semiconductors are essentially the
same. In contrast, SnO is easily accessible, absorbs a sizeable
amount of solar energy, and has higher photo catalytic activity
than TiO2.
Due to their exceptional characteristics, such as low cost,
photoconductive response, pyro electricity, and surface
fictionalization, high binding energy, and electron transfer
efficiency, zinc oxide based materials are used in
multifunctional electrode applications such as dye sensitized
solar cells, lithium ion batteries, gas sensors, air quality monitoring. These materials are likewise inexpensive,
inexpensive, and This metal oxide based semiconductor is
used in several different optoelectronic technologies,
including light emitting diodes, flat panel displays, transparent
semiconductors, and conductive oxides, due to its exceptional
optical properties [1-5].
The studies have examined the use of SnO based materials for
sensing and photovoltaic applications, including the detection
of ammonia gas using Ag/SnO flowers and Cu doped SnO
nanostructures, the detection of NO2 gas using tiSnO thin
films, the detection of ethanol vapour using SnO. In 2O3 core
shell nanofibers, and the development of quaternary
transparent conductive oxide materials lithium ion batteries
have proven a major success in little electrical devices.
To create pure NiO nanoparticles, it is necessary to regulate
the solution's pH level, structure, and calcinations
temperature. These factors have an impact on the particles'
shape, size, and dispersion. If pure NiO is created, its precise
physical and chemical characteristics can be identified.
Because of its superior crystalline, uniform particle dispersion,
homogenous mixing, and smaller particle size, the sol gel
process is a good choice for making NiO nanoparticles [6].
The use of lithium in massive electrical energy storage devices
is nonetheless constrained by the dearth of lithium minerals.
Layered sodium transition-metal oxides are promising
materials that can significantly aid in the development of
large scale electrical energy storage devices by reducing the
issues associated with lithium batteries due to their
exceptional cycle stability and rate performance [7].
SnO, on the other hand, has large bandgap energy and only
absorbs electromagnetic radiation in the UV area. Because
just 3%-5% of the solar energy's Ultraviolet (UV)
component reaches the earth, its photo catalytic activity
under solar radiation is limited. In order to increase the
photocatalytic activity of existing photo catalysts like SnO,
doping or co-doping with metals and nonmetals is
currently being used. Additionally, exchanging charge
carriers amongst several nanostructured semiconductors
enhances photo catalytic performance [8].
Consequently, there were national strategies for the
evaluation of the clinically relevant nanoparticle
presentations. We want to highlight the function and
efficiency of nanoparticle usage in cancer treatment. In this
review, as well as the significance of toxicity testing for this
novel strategy (Figure 1).
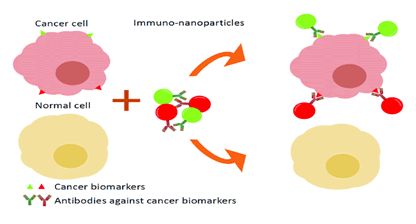
Figure 1: A good illustration of the distinction
between passive and active nanoparticles in cancer
tissue.
As a photocatalyst for visible light, Cuprous Oxide (CuO),
a tapered band gap semi-conductor, has been proposed.
Using the energy from visible light, electrons in CuO can
transition from the valence band to the conduction band.
However, after being formed, electrons and holes generated
by lasers mix fast, which might harm the photocatalytic
activity. The CuO has previously been combined with
grapheme and other metals to prevent photo induced
electrons and holes from recombining. It is anticipated that
CuO will be employed in tandem with high band gap metal
oxides, such as SnO, as an operational strategy to
address the charge carrier recombination issue. In this
work, a CuO/SnO nanocomposite was made using coprecipitation
and thermal breakdown procedures in the
eradication of the model dye pollution methyl red (Figure
2) was examined.
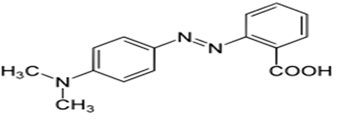
Figure 2: Structure of methyl red.
It ranks as the third most frequent cancer among women and
the fourth most frequent reason for cancer related
mortality. 16,710 new cases, or 16.35 occurrences per 100,000
women, are anticipated in 2022. With a mortality rate of
5.33 per 100,000 women, Cervical Cancer (CC) claimed
6,627 lives in the nation in 2020. In 2019, CC resulted in a
loss of 160.8 DALYs.
Materials and Methods
Experimental Study
Sample preparation: The chemicals used included metal
oxides, solvent NaoH, copper, nickel, and tin chlorides. The
sol gel approach made use of analytical grade chemicals.
Distilled water was used in every experiment. The 1 M tin
chloride was mixed with 30 ml water. 1 M NaOH was mixed
with 30 ml water in the same way. Then, drop by drop, add
the NaOH solution to the tin chloride solution. After 20
minutes of stirring, gel formation was obtained. Then place it
in the microwave oven at the temperature specified. Then,
obtain powder and thoroughly mill it to obtain nano powder.
Characterization techniques: The crystal structures
and average crystallite size of the samples were determined
using an XRD (Shimadzu XRD-7000, Shimadzu corp., Kyoto,
Japan) in phase scan mode with Cu-K radiation (=0.15406
nm), phase time and degree (2) of 0.4 s and 0.02 s,
accordingly, for the range of 10 to 80. The surface
morphology of the materials was examined using a JEOL
JSM-5610 Scanning Electron Microscope (SEM) with an
Everhart-Thornley detector (JEOL, Ltd., Akishima, and
Tokyo). Utilizing KBr pellets and an FT-IR spectrum 65 (PerkinElmer, Waltham, MA, USA) in the 4000 cm-1-400
cm-1 range, the chemical makeup of the generated samples
was determined. The absorption spectra were
estimated using the Kubelka-Munk method, and the
properties of the nanocomposites were examined using a
PerkinElmer Lamda 35 spectrometer with a 200 nm-800 nm
wavelength range [9-14].
Results and Discussion
XRD Analysis
The nano-particle diffraction pattern is consisting of CuO,
SnO, CuO/SnO, and CuO/SnO are shown in Figure 3. Similar
results were reported by diffraction peaks of SnO, and CuO/
SnO, nanomaterial’s found in XRD patterns at 2=31.76, 34.40,
36.24, 47.53, 56.59, 62.85, 66.37, 67.90, and 69.07, which
correspond to the (100), (002), (101), (102), (110), (103),
(200), (112). According to Jiang, et.al., the cuprous oxide
XRD patterns diffraction peaks at 2 values of 29.57, 36.40,
42.32, 61.43, 73.55, and 77.40 are reflections from the (110),
(111),(200), (220), (311) and (222) crystal planes. XRD was
used for further investigation to validate the phase of the
produced NiO nanoparticles. The material was in an
amorphous phase prior to calcinations, and no NiO phase
was seen. All of the NiO diffraction lines were indexed to an
ordered structure in the cubic structure that the crystalline
phase displayed after the calcinations. All of the diffraction
peaks at (111), (200),(220), (311), and (222) were seen at the
crystalline phase.
The absence of additional diffraction peaks caused by
contaminants like CuO, Cu (OH), demonstrates the purity of
the nanostructured materials. Figure 1 demonstrates that
doping did not produce an additional peak, suggesting that
the SnO and CuO lattices. The little shift in the NiO
nanocomposite's diffraction peaks.
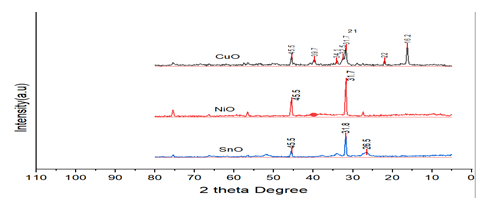
Figure 3: X-ray diffract gram of CuO, NiO and SnO of nanomaterials.
The scherer equation was used to determine the typical
nanomaterial crystallite sizes from the strong peak. Here, D
stands for maximum size at full width, wavelength, bragg
angle, and crystallite size in radians. Crystallite diameters for
SnO, CuO, and NiO were reported to be 33.72 nm, 32.33 nm,
and 13.57 nm, respectively. Considering these outcomes,
nanocomposite has smaller crystallite sizes than CuO and
SnO. The fact that NiO nanocomposite crystallite size is
smaller than CuO and SnO nano-level particles may be due to
the disruption of particle formation caused by CuO and SnO
lattices.
SEM Analysis
As seen in the use of SEM was examining the surface
morphology of nanomaterials made of CuO, SnO, and NiO.
According to SEM micrographs, SnO has some agglomerated
nanoparticles with an irregular shape, which is in line with.
But SEM images of CuO, SnO, and NiO samples showed that
they were reasonably organized and that there were much
less particle agglomerations than in SnO nanoparticles with
truncated octahedron shaped nanocrystals; this could be
because CuO was present (Figure 4).
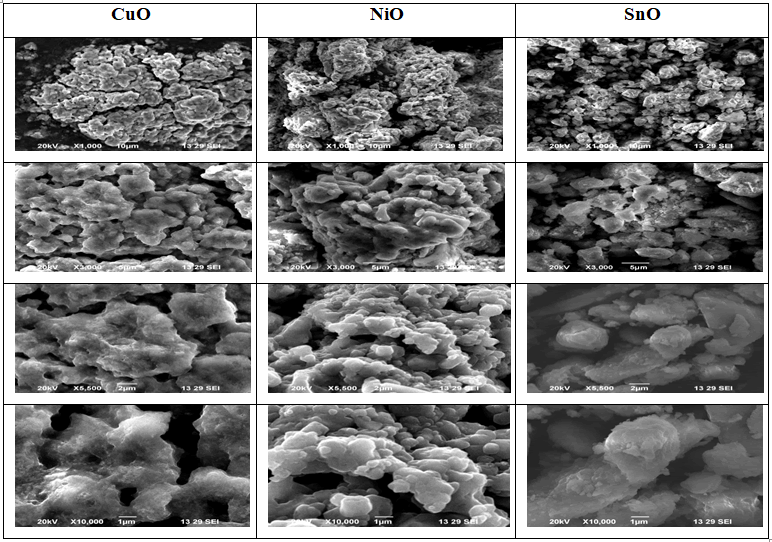
Figure 4: Scanning Electron Microscopy (SEM) morphology
of nanomaterials.
FTIR Analysis
The FTIR bands of CuO, SnO, and NiO nano-materials are
shown in Figure 3. With the exception of CuO, all materials
showed peaks that appeared in the 490 cm-1-505 cm-1 regions. These peaks correlate with the stretching vibration of
SnO and match up with earlier studies. Peaks in the FTIR
spectra of CuO, SnO, and NiO nanoparticles were observed
and match the stretching vibration of CuO, with peaks in the
region of 610 cm-1-630 cm-1; a related discovery was
reported by the N-H stretching vibration mode may be the
cause of the peak at 3169 cm-1 in the FT-IR spectra of the NiO
nano-composite. An N-H bending vibration mode may be the
source of the band at 1441 cm-1. The FT-IR spectra of the
CuO/SnO nano-composite showed the aforementioned
absorption bands in addition to a peak at 431 cm-1, which
may be caused by the stretching vibration since similar results
have been reported. As a consequence, the bands
demonstrated that the NiO nanocomposite included SnO,
CuO, and NiO (Figure 5) [15].
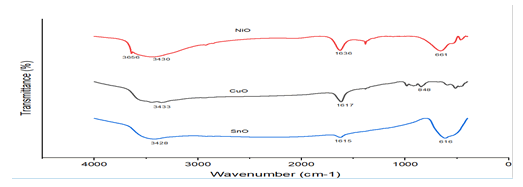
Figure 5: Fourier Transforms Infrared (FTIR) spectrums of CuO,
SnO, and NiO nanomaterials.
Cell Procession
The HeLa (human cervical cancer cell line), which was grown
in eagles least essential medium with 10% foetal bovine
serum, was given by the NCCS (National Centre for Cell
Science), Pune. The conditions for the cells' maintenance
were a constant 370°C, 5% CO2, 95% air, and 100% relative
humidity. Maintenance cultures were tested every week, and
the culture medium was changed twice a week [16].
Process for Treating Cells
The monolayer cells were separated into individual cell
suspensions using EDTA acid (Trypsinethylenediaminetetraacetic
acid), and viable cells were
measured using a hem cytometer. Then, using medium
containing 5% FBS, the specific cell suspensions were diluted
to a final cell density of 1 × 105/ml. 10,000 cells in 100 micro
liters of cell solution were seeded on 96 well plates, and they
were incubated to encourage cell adhesion at 370°C, 5% CO2,
95% air, and 100% relative humidity. After a 24 hour period,
increased dosages of the test substances were administered
to the cells. In the following step, a sample solution was
created and diluted using serum free medium to double the
required final maximum test concentration. To spread them,
they were originally welcomed into Phosphate Buffered Saline
(PBS). To produce a total of five sample concentrations, four
further serial dilutions were carried out. These different
sample dilutions were added to the appropriate wells that
already had 100 l of medium in order to produce the necessary final sample concentrations. The
plates were incubated for 48 hours after the sample was
added at 37°C, 5% CO2, 95% air, and 100% relative
humidity. For each concentration, three duplicates were
retained, and the control medium was used [17].
MTT Assay
3-[4, 5-dimethylthiazol-2-yl] yellow, water soluble tetrazolium
salt 2,5-diphenyltetrazolium bromide (MTT). The
mitochondrial enzyme succinate dehydrogenase breaks the
tetrazolium ring, converting the MTT into an insoluble purple
formosan. Thus, the quantity of formosan generated and the
number of viable cells is directly correlated. Each well
received 15 l of MTT (5 mg/ml) in Phosphate Buffered Saline
(PBS), which was added, and was then incubated at 370°C for 4
hours a ter the initial 48 hours of incubation. When the MTT
medium was stopped, the formosan crystals were dissolved in
100 l of DMSO, and the absorbance at 570 nm was then
quanti ied using a micro plate reader. The formula below was
used to determine the percentage of cell inhibition [18-20].
% Cell Inhibition=100-Abs (sample)/Abs (control) × 100.
The IC50 was determined by creating a nonlinear regression
graph between the percentage of cell inhibition and the log
concentration using the graph pad prism tool (Table 1, Figures
6 and 7).
| HeLa |
Conc. |
6.25 µg |
12.5 µg |
25 µg |
50 µg |
100 µg |
Cont |
|
|
| E 01 |
ABS |
0.531 |
0.525 |
0.33 |
0.237 |
0.032 |
0.607 |
IC50 |
33.54 µg/ml |
| |
0.566 |
0.489 |
0.367 |
0.248 |
0.037 |
0.596 |
|
|
| |
0.559 |
0.503 |
0.359 |
0.244 |
0.054 |
0.587 |
R² |
0.977 |
| Avg |
0.552 |
0.507 |
0.352 |
0.243 |
0.041 |
0.596 |
|
|
| % Cell inhibition |
7.486 |
15.251 |
41.005 |
59.273 |
93.128 |
|
|
|
Table 1: Data for MTT Assay
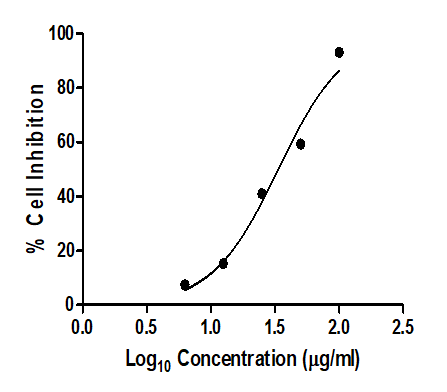
Figure 6: Data for MTT Assay.
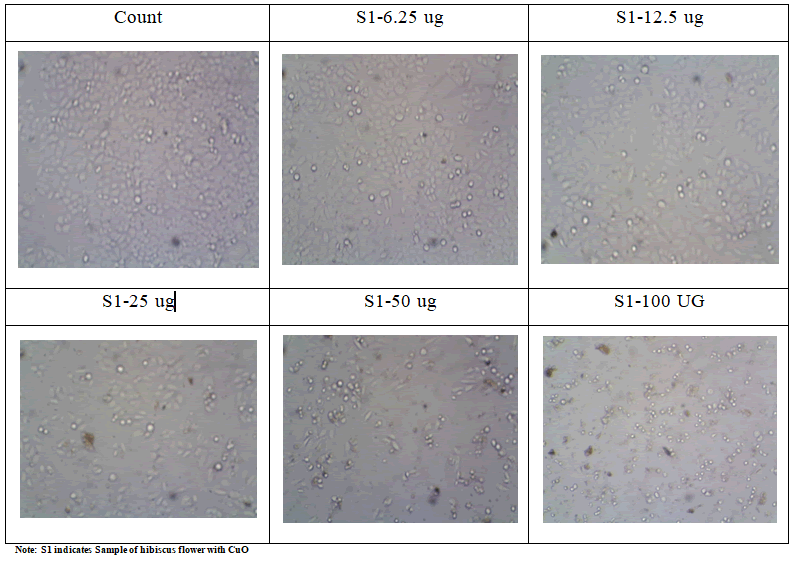
Figure 7: Plots for MTT Assay.
Conclusion
The fabrication of a CuO/SnO nano-composite used coprecipitation
and thermal breakdown techniques. The
NiO nanoparticles were successfully synthesized through the
sol gel method. Thermal analysis was used to estimate the
NiO nanoparticles' ideal calcinations temperature. The
NiO nanoparticles developed a cubic structure. The
sample demonstrated that no impurities were present, just
pure NiO nanoparticles. The nanoscale range of the
morphological analysis was proved, and NiO elements
were effectively identified. In comparison to SnO,
CuO, and CuO/SnO nanoparticles, the absorption edge of
the nanocomposite was more extended to the visible
range of electromagnetic radiation. Connection of CuO and
SnO semiconductors, which may lower charge carrier
recombination rates and boost solar absorption and use, is
responsible for the improvement. CuO, NiO, and SnO
nanocomposites showed MIC90 antifungal activity in a
research against vaginal isolates of C. albicans. Cervical
cancer is still a severe illness in less developed nations
and regions, where effective preventative efforts still face
considerable obstacles. The disease burden has fallen dramatically in industrialized countries and regions over the
past several decades, but it is still a problem there. Various
cost effective and scientifically supported preventative and
control techniques are already available to fulfill the demands
of regions with varying economic levels.
References
- Agarwal H, Kumar SV, Rajeshkumar S (2020) Antidiabetic effect of silver nanoparticles synthesized using lemongrass (Cymbopogon citratus) through conventional heating and microwave irradiation approach. J Microbiol Biotech Food Sci. 9(6):371-376.
[Crossref] [Google Scholar]
- Al-Zoubi MS, Aljabali AA, Pal K (2021) Highly toxic nanomaterials for cancer treatment. Bio man Nano. 18:161–185.
[Google Scholar]
- Alomari G, Al-Trad B, Hamdan S, Aljabali A, Al-Zoubi M, et al. (2020) Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy. Drug Deliv Trans Res. 10 (1):216–226.
[Crossref] [Google Scholar] [PubMed]
- Al-Trad B, Alkhateeb H, Alsmadi W, Al-Zoubi M (2019) Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat diet/streptozotocin induced diabetic rat. Life Sci. 216:183-188.
[Crossref] [Google Scholar] [PubMed]
- Arvanag FM, Bayrami A, Yangjeh AH, Pouran SR (2019) A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Mater Sci Eng C. 97:397–405.
[Crossref] [Google Scholar] [PubMed]
- Balcha A, Yadav OP, Dey T (2016) Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ Sci Pollut Res. 23:25485–25493.
[Crossref] [Google Scholar] [PubMed]
- Behnajady M, Modirshahla N, Hamzavi R (2006) Kinetic study on photocatalytic degradation of CI acid yellow 23 by SnO photocatalyst. J Hazard Mater. 133:226–232.
[Crossref] [Google Scholar] [PubMed]
- Benedix R, Dehn F, Quaas J, Orgass M (2000) Application of titanium dioxide photocatalysis to create self-cleaning building materials. Lacer. 5:157–168.
[Google Scholar]
- Bora LV, Mewada RK (2017) Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew Sustain Energy Rev. 76:1393–1421.
[Crossref] [Google Scholar]
- Cauda V, Pugliese D, Garino N, Sacco A, Bianco S, et al. (2014) Multi-functional energy conversion and storage electrodes using flower like zinc oxide nanostructures. Energy. 65:639–646.
[Google Scholar]
- Chen T, Liu W, Zhuo Y, Hu H, Zhu M, (2020) Single phase P2-type layered oxide with Cu-substitution for sodium ion batteries. J Energy Chem. 43:148–154.
[Google Scholar]
- Cheng CA, Deng T, Lin FC, Cai Y, Zink JI (2019) Supramolecular nanomachines as stimuli-responsive gatekeepers on mesoporous silica nanoparticles for antibiotic and cancer drug delivery. Theranostics. 9(11):1-24.
[Google Scholar]
- Emeline A, Ryabchuk V, Serpone N (2007) Photoreactions occurring on metal oxide surfaces are not all photocatalytic: Description of criteria and conditions for processes to be photocatalytic. Catal Today. 122:91–100.
[Google Scholar]
- Essawy AA (2018) Silver imprinted zinc oxide nanoparticles: Green synthetic approach, characterization and efficient sunlight induced photocatalytic water detoxification. J Clean Prod. 183:1011-1020.
[Google Scholar]
- Gionco C, Fabbri D, Calza P, Paganini MC (2016) Synthesis, characterization, and photocatalytic tests of N-doped zinc oxide: A new interesting photocatalyst. J Nanomater. 16:1-8.
[Crossref] [Google Scholar]
- Gupta SM, Tripathi M (2012) An overview of commonly used semiconductor nanoparticles in photocatalysis. High Energy Chem. 46:1–9.
[Google Scholar]
- Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling an overview. Rsc Adv. 2:6380–6388.
[Crossref] [Google Scholar]
- Huang B, Zhang Z, Zhao C, Cairang L, Bai J, et al. (2018) Enhanced gas sensing performance of SnO. In 2O3 core@ shell nanofibers prepared by coaxial electrospinning. Sens Actuators B Chem. 255:2248–2257.
[Crossref] [Google Scholar]
- Jang JS, Kim J, Ghorpade U, Shin HH, Gang MG, et al. (2019) Comparison study of SnO based quaternary TCO materials for photovoltaic application. J Alloy Compd. 793:499–504.
[Crossref] [Google Scholar]
- Jiang D, Xing C, Liang X, Shao L, Chen M (2016) Synthesis of cuprous oxide with morphological evolution from truncated octahedral to spherical structures and their size and shape-dependent photocatalytic activities. J Colloid Interface Sci. 461:25-31.
[Crossref] [Google Scholar] [PubMed]
Citation: Devi VA, Kalaiselvi V (2023) Nanoparticles Reactions in Cervical Cancer: Challenge and Hope. J Nanosci Nanotechnol Res. 7:012.
Copyright: © 2023 Devi VA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.