Keywords
Stromal cells; Pluripotency; Reproducible; Multiorgan engraftment; Striking spectrum; Prosecutions; Tissue mending
Abbreviations
MSCs: Mesenchymal Stem Cell; BM: Bone Marrow; HSCs: Hematopoietic Stem cells; CFU-Fs: Colonized fibroblastic units; GVHD: Graft Versus Host Disease; AT: Adipose Tissues; EPCs: Endothelial Progenitor Cells; BMP: Bone Morphogenic Protein; NEBL: Nebulette; LPL: Lipoprotein Lipase Genes; qRT PCR: Qualitative Real Time-PCR; Runx2: Runt-Related Transcription Factor; IBMX: Isobutyl Methyl Xanthine; GNDF: Glial Cell Lines Derived Neurotrophic Growth Factors; HNF-4α: hepatocyte nuclear Factor; ZNF: Zinc Finger Protein; PDGFR: Platelet Derived Growth Factor Receptor; LVEF: Left Ventricular Ejection Fraction; CNS: Central Nervous System; DCs: Dendritic Cells; CM: Conditioning Media; CARCs: CXCL Abundant Reticular Cells
Introduction
Mesenchymal stromal cells stem cells (MSCs) have elucidated the global and scientific attention mainly due to their potential to harbor the self-renewal and regenerative capability during repairing, limiting and reconstituting the respective tissue components in bone regeneration phenomenon [1]. Being characterized as heterogenous, plastic adherent, distinct stromal sub-population, MSCs having the lineage commitment capability is an active field of investigation in the world of stem cell based innovations [2].
After HSCs discovery, research work of Friedenstein testified the presence of non- hematopoietic stromal cells with adipose and skeletal potential observed during heterotopic transplantation that can form reticular cells, cartilage, fat cells and bone [3]. With passing years, researchers have extended and confirmed these observations by isolating them from bone marrow (BM) and by elaborating the passaging and induced differentiation procedures of MSCs under the in vitro cultured conditions into multiple mesodermal and non-mesodermal lineages [4,5].
Progressive researches in field of MSCs has revealed the MSCs niche component and it has explained that though bone marrow is direct source of isolation of MSCs but it is also found in post-natal organs considering from the birth tissues to skeletal muscles and even dental pulp. However, the frequency and number of MSCs present in different tissues can be varied depending on the corresponding tissue type [6-8].
Significant novel methods of immunoselection, enzymatic and non-enzymatic degradation and analytical PCR has been performed for analyzing their phenotypic characteristics, cells expansion, and differentiation in vitro as well the recognition of factors that served to play part during their in vivo transplantation [9,10]. Various evidences have suggested that many signals transducing networks and interacting pathways are involved in regulating the differentiation of MSCs. Due to significant clinical advancement of MSCs in field of medicine, it is considered to be imperative to expose pathways adapted by regulators with which MSCs undergone differentiation and discovery of micro RNA dependent lineage commitment has provided a new insight in understanding the mechanisms regulating their differentiation [11,12].
Operationally, all the findings about MSCs is virtually dependent on their in vitro characterization and a very little has been cited about their in vivo counter partner. Therefore, criteria for defining MSCs is actually on basis of their in vitro self-renewal and differentiation potential [13]. Lately, an effort has been done in explaining their anatomical origin and it has shown their homology with in vivo residing pericytic cells surrounded by blood vessels [14]. Additionally, no defined in vivo assays have been identified so far that can explain its unique marker of stemness, yet, recent studies has suggested the in vivo role of MSCs in maintaining the hematopoietic environment and stability of the bone marrow compartment [15].
An important reason to investigate the origin and underlying differentiation mechanism of MSCs is to enhance their therapeutic potential in preclinical and clinical trials. Scientists have made advancement for analysis of the healing benefits of MSCs according to their biological functions and they have concluded that certain growth factors, soluble secretory molecules and paracrine factors are involved that engage the recipient cells during tissue repair process. Currently, main goal of scientific innovations in study of MSCs is there in vivo characterization as it can lead to isolation of enriched pure stromal cells population that can be used for organogenesis, tissue engraftment and immunomodulatory effects which will renovate the world of clinical cell mediated therapies [16].
Literature Review
This brief review provides a brief understanding about MSCs spotted right from their original perception and conception to its therapeutic properties focusing their in vivo and in vitro regulatory mechanisms of differentiation into multiple lineages and their organizing roles in the clinical biology.
Origin and Perception of Mesenchymal Stem Cells
Cohnheim, in 1867, was the first scientist who hypothesized that a bone marrow (BM) source of the fibroblasts exists and played its role during the wound healing process. Followed by this observation, Maximow described a vital link among freshly forming blood constituents and mesodermal cells during the process of embryogenesis. He postulated the role of stromal marrow in subsiding the progression and maintenance of blood with hematopoietic organs. These observations done by both scientists presented the very first idea about the existence of stromal cells population in BM that served to be involved in natural healing process and hematopoiesis [17,18].
In early 1960s, in vivo BM transplantation demonstrated the role of these precursors as formers of cellular tissues. Transplanting these stromal cells in subcutaneous layer or just beneath the kidney capsule led strangely towards growth of ectopic marrow. Decomposing the origins of these ectopic marrow cells, it exposed the bony trabeculae of donor, adipocytes, myeloid supportive stroma and host derivatives of hematopoietic stem cells that formed the colony and undergo maturation inside the lumen [19,20]. Friedenstein et al. were first who verified that stromal cells possibly be secluded from BM based on their ability to adhere differentially with tissue culture plastic technique, still used for MSCs isolation. These cells were firstly regarded as non-phagocytic and adherent cells having ability to form colony forming units that are fibroblastic in nature [21]. Experiments made with connective tissues and dermal fibroblasts failed to retrieve the similar histological demonstration as made by when stromal cells were transplanted in kidney capsule or other tissues, representing it a marrow stroma specific phenomenon. These pivotal experiments proved that MSCs has similar function like lymphoid stromal cells and recommended MSCs to be the predecessors of BM connective tissues. Lately in 1980s, Maureen Owen with Caplan demonstrated the work of Friedenstein and proposed about the presence of adult stem cells, considered to be responsible for process of mesengenesis. Further, Owen et al. characterized these marrow stroma cells and also illustrate their heterogenicity [22,23]. Meanwhile, Caplan and his colleagues theorized that subpopulation of stromal cells was developmentally linked with mesenchymal tissues which they had been analyzing in the process of chick embryogenesis. Moreover, scientists identified the first MSCs expressed antigens which show a reaction with monoclonal antibodies at SH2 (CD105) and SH3 (CD73) subsequently. He formulated the name “mesenchymal stem cells” for describing that subtype of stromal cells which were found to be involved in mesengenesis [23-25].
Presence of a stromal precursor differentiating into mesodermal cell lines in BM was initially theorized in nineteenth century [26]. Succeeding towards approaches used for isolation and culturing of MSCs, this field began its progress rapidly and many scientists began to discover their therapeutic practices. Few years after the discovery of MSCs, human efforts were inaugurated to assess the safety and effectiveness of MSCs mediated therapy. Primarily, autologous MSCs were investigated to aid in tissue engraftment and retrieval of hematopoiesis after ablation and marrow transplantation performed in treatment of cancer [27,28]. Concurrently, researchers conducted a series of innovative experiments to investigate about therapeutic probabilities for allogeneic MSCs transplants in treatment of children suffering from osteogenesis imperfecta which is a genetic disease of skeletal dysplasia [29]. Thereafter, further investigations were made for treating patients suffering with Hurler syndrome and metachromatic leukodystrophy with help of allogenic MSCs. The results of these investigations were very significant as it provided evidence for safety of MSCs mediated therapies [30].
Lately, International Society for the Mesenchymal Tissue and Stem Cell Committee projected the minimal criteria for defining human MSCs (hMSCs). Firstly, hMSCs must be plastic adherent under standard cultured environment and must form colonized fibroblastic units (CFU-Fs). Secondly, hMSCs must exhibit CD73, CD90 and CD105 markers expression and absence of CD14, CD11b, CD19, CD34, CD45, CD79 and HLA-DR molecules. Lastly, hMSCs must differentiate into chondroblasts, osteoblasts and adipocytes in vitro [31].
Currently, on basis of recent innovation made about pleotropic function of MSCs that favors to attenuate immune dysfunction as well as enhancing endogenous repairment, MSCs transplantation has been investigated for treating myriad disease. Presently, registered experimental sites are present for assessing MSCs therapy all over the world, signifying global curiosity for exploring MSCs as a potential therapy source. Most of the trials are funded by academic medical centers for exploring novel features of MSCs in diverse areas as liver cirrhosis, spinal cord injury and critical limb ischemia. Osiris Therapeutics, instituted in 1991, has played an influential role in past decade in promoting the research and expansion of MSC based clinical therapies. Based on researches made on immunological role of MSCs, it has introduced a clinical trial to explore the therapeutic efficacy of MSCs in humans. It has pioneered the studies to investigate the administration of MSCs as a therapy for type 1- diabetes mellitus, crohon’s disease, myocardial infraction, graft-versushost diseases (GvHD) and chronic obstructive pulmonary disorders (COPD). A good safety record for MSCs injection has expanded the MSCs mediated clinical trials in Phase II and Phase III and currently MSCs have been approved as expanded access to be used in pediatric refractive GvHD [32-34]. However, molecular signature and their in vivo status of proliferation remain unclear and is subjected to investigation, though the ex vivo cultured MSCs are being extensively used in several studies [35,36].
Many clinical trials entail the administration of MSCs systemically and assume that MSCs engraftment provide long term support by either directly replenishing damaged tissue or interacting with neighboring cells to favor the endogenous repair. Currently, it is widely debated whether MSCs engraftment, proliferation, or differentiation is obligatory for therapeutic benefit [37]. Many recent studies are now predicting the paracrine signaling as the primary mechanism of action followed by soluble factors secreted by MSCs and a few studies have even demonstrated that direct injection of the molecules secreted by MSCs can provide an improved benefit when transplanted to the whole cells [38].
MSCs Niche evidences from several tissues
BM derived MSCs, due to their ease in accessibility, multipotent nature and expandability, hold significant potential for their role in the regenerative medicines as well as tissue engineering [39]. However, besides the BM, well recognized sources are also reported in body for MSCs. Probably, primary MSCs population can be isolated from fetal birth tissues like Wharton’s jelly, umbilical cord matrix and umbilical cord blood. It is worthy to note that umbilical cord blood matrix serves more good source of MSCs than umbilical cord blood [40]. Wharton’s jelly mainly contain primitive MSCs thus upsurges their potential in therapeutic submissions [41]. Additionally, amniotic fluid has also been revealed to contain MSCs and it can be attained with amniocentesis or during the birth time.
Other birth related tissues like amnion and placenta though contain MSCs, however due to their quantity and regarding the fact that they hold a heterogenous hematopoietic stem cells (HSCs) population and endothelial progenitor cells (EPC), their potential therapeutics differs from the pure MSCs [42]. With growth, the next possible young cell source is the developing dental pulp which is easy to collect among 8-10 of ages in children. There is an ambiguity if these cells serve to be pluripotent or multipotent, however, they are proposed to be a key source for cell banking in future and have potential to replace usage of umbilical cords [43].
In adults, Adipose tissues (AT) are found to be direct source of MSCs, which is simply accessible and a well-defined methodology is present that favors their isolation from source. It is assessed that round about 500 times, more in number, AT derived MSCs can be isolated more easily from fat tissues comparative to the BM of same amount [44]. Moreover, mobilized peripheral blood also serve as a source of MSCs as well, anyhow, conceded by several aspects related to the donors like sex, age, feeding habit, administered drugs, daytime and health status. It has been used in cartilage repair and efficient results are observed [45].
Efficient population of MSCs has been reported in menstrual blood and endometrium (Figure 1), that are found to be exhibiting stem cell like marker and phenotypes and are found to show neuroprotection in a stroke condition [46,47]. Skin and fore skin are also source of MSCs and evidenced to be differentiated into multiple lineages. [48].
Figure 1: A flow representation of Mesenchymal Stromal Cells (MSCs). MSCs are reported to find in various tissue sources in the body and can be isolated from them. MSCs are found in endometrium, adipose tissues, cartilage, dental pulp, bone marrow, neuronal cells, placental tissue and skin and foreskin.
Characterization and Potency Analysis of MSCs in vitro:
Prospective isolation techniques for MSCs
Application of MSCs in clinal trials require proper isolation, culturing and the differentiation into subsequent lineage. Since time of its discovery, a density gradient centrifugation method was implied to separate the mononuclear cells and red blood cells in BM unless it was potentiated by percoll gradient separation and ficoll gradient separation [49]. Recent investigation has suggested that percoll density separation method is best in term of providing adequate yield of MSCs whereas as ficoll protocol is reported to cause extensive graft loss and less productive yield of MSCs [50]. The latest innovation involves invention of automated, closed system that supervise the processing of MSCs from its collection to its application in clinical therapy. This automated system has been applied extensively on broad scale for AT derived MSCs [51].
Some studies, on clinical scale, adapted direct plating approach to separate the plastic adherent cells from nonadherent ones. In presence of optimal conditions ficoll, percoll and whole BM was directly plated and compared for the same samples to check efficiency of separation methods. Optimized Seeding densities are considered as the most crucial factor while isolating and expanding MSCs. After attaining confluence, cells were replenished after detaching and were plated once again. Results suggests that plating of whole marrow directly is more advantageous in term of cell growth, CFU-Fs number along with minimal manipulation. The challenge is to establish a compromise with both cell quality and expansion along with cost issues in clinical scale expansion of MSCs [52].
One of conventional method for isolation of hMSCs involves enzymatic digestion method by using collagenase enzyme for isolation of human adipose derived MSCs. After enzymatic digestion and the preliminary adherence phase, nonadherent cells are discarded and the remaining adherent ones are appeared as fibroblastic cells. Now it has been replaced by explant derived MSCs isolation process. In this method, small pieces of human AT derived MSCs were placed in a culture flask with suitable medium elements via liposuction and is kept being incubated. Homogeneity of the isolated cell population, high yield and less time consumption make it advantageous over conventional enzymatic degradation method [9,53].
Immunoisolation is another method for isolation of MSCs before culturing on basis of cell surface markers. It is actually the positive selection technique performed by using monoclonal antibodies cross reacting against MSCs markers [54]. Moreover, immunodepletion is another negative selection method where MSCs population is supplemented by washing after being labeled with the antibodies, directed against mostly the hematopoietic markers. It is actually purifying method of MSCs from hematopoietic contamination. At early stages of passaging, MSCs can be isolated and purified from hematopoietic cells by immumodepletion of hematopoietic cells [55]. Recently, more particular and pure MSCs populations can be isolated utilizing a combinatorial method of immunoisolation along with immunodepletion based on the surface markers. The novel technique for isolation of non-cultured MSCs evolved includes affinity-based methods, microscale technologies as well as biophysical properties-based methods demanding no labelling of cells followed by suspending under fluid conditions [10].
Culturing analysis of MSCs
MSCs after being successfully isolated from different sources, are cultured using condition media namely Dulbecco modified Eagle media (DMEM) and RPMI (Roswell Park Memorial Institute medium) with minimum essential medium alpha (MEM α) mentioned to be one of the best medium for culturing of MSCs in vitro [56]. Dexamethasone is considered to be leading factor in culture medium for inducing osteogenic differentiation while bone morphogenetic protein BMP2 provide supportive properties when added in culture media along with vitamin D3 and fibroblast growth factor [57]. One of the major challenge during culturing is to obtain sufficient number of cells as it has been observed that starting fresh tissue culture available is in limited amount and on subculturing, these cells showed a decline in potency along with time consumption problems and genetic instabilities that results ion MSCs aging and senescence. Currently an advancement has been made by establishing an evaluation of a new cell bank strategy that has resulted in MSCs cell lines with stable genotypic and immunophenotypic characters [58].
Differentiation of MSCs into multiple lineages
hMSCs have the ability to differentiate by in vitro high capacity assays into all three i.e. endodermal, ectodermal and mesodermal lineages that confirms their self- renewal and multipotent ability [59].
Mesodermal lineages: The in vitro differentiation potential of MSCs into mesodermal lineages evidenced by performing qRT PCR (qualitative real time-PCR), flowcytometry and cytochemical analysis has been evaluated [7,60]. MSCs differentiation into adipocytes is induced by providing appropriate media supplementations, which stimulates the activation of transcription factors and genes responsible for adipogenesis. Cultured MSCs in growth medium supplemented with growth factors were analyzed for adipogenesis by expression of adipocytes specific genes, lipoprotein lipase genes (LPL), adipocyte protein ap2 and peroxisome proliferator activated receptor PPAR γ [61,62]. Induction of MSCs towards adipogenesis is a two-phase process. During the determination phase, cells are committed towards the preadipocytes, are morphologically similar to fibroblasts and eventually are not distinguished from MSCs precursors. At terminal phase, the pre-adipocytes form the mature adipocytes along with lipid droplets and also express the adipocytes particular proteins. Overall, adipogenesis is an sequential ordered process with multiple signaling pathways [63].
Osteogenic induction of MSCs is characterized by minerals aggregation and an increase in enzyme activity of alkaline phosphatase in final stages of differentiation. These mineralized aggregates were found to be positive for von kossa staining and Alizarin Red [64]. The progression of osteogenesis initiates with presence of osteoprogenitors which at early stages, differentiate towards pre-osteocytes and then latterly into mature osteoblasts. Factors that are involved in the induction of osteogenesis includes activation of runt-related transcription factor-2 (Runx2) along with bone morphogenic protein BMP 9, and many other extracellular signaling molecules [65,66]. In the bone formation process, osteoblasts firstly synthesize the bone matrix and then allows the mineral deposition and bone remodeling [67]. Many researchers have stated that a relationship exist between osteogenesis and adipogenesis. Several signaling cascades are demonstrated to be the positive regulators of osteogenesis and negative regulators of adipogenesis. Signaling pathways like NEL-like protein, Hedge Hog and Wnt pathways are well demonstrated for anti adipogenic and pro-osteogenic inductions in MSCs [68-70].
hMSCs also have the potential to differentiate towards chondrocytes lineages. In final step, pre-chondrocytes differentiate towards the mature chondrocytes and show an expression of chondrogenic transcription factors like Sox5, Sox6 and Sox9 [71]. Along with transforming growth factor TGF-β1, insulin like growth factor-I and BMP-2 also contributed towards chondrogenic differentiation of MSCs. In hMSCs, growth factor TGF-β1 interacts to Wnt β-catenin dependent pathways and hinders the osteoblast differentiation and induces the chondrogenesis. Apart from the growth factors, parathyroid hormone related peptides and triiodothyronine T3 also influenced the induction of chondrogenesis [72,73].
Ectodermal lineages: On providing neural induction culture media along with growth factors cocktail, like retinoic acid, brain derived neurotropic factors, isobutyl methyl xanthine (IBMX) and Glial cell lines derived neurotrophic growth factors (GNDF), expanded MSCs transdifferentiate towards neuronal cells exhibiting particular neuron specific phenotypes. The induced neuronal cells were found to express Nestin, neuronal nuclear protein NeuN, dopamine transporter protein DAT and tyrosine hydroxylase protein TH, neuronal potential of differentiation [74]. Normally an enhancer or Zest Homolog type 2 controls the stem cell proliferation, maintenance and eventually differentiation into multiple lineages involving neurons. It is noted that changes observed in concentration of intracellular calcium plays a key role in induced neuronal differentiation of MSCs [75]. Gangliosides, a class of glycosphingolipids, when interact with epidermal growth factor receptor is reported to enhance the osteoblast formation (Figure 2).
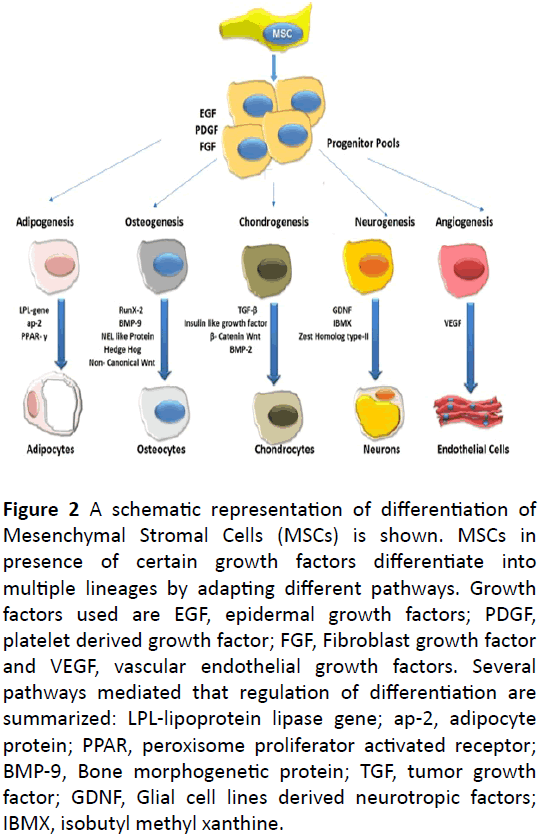
Figure 2: A schematic representation of differentiation of Mesenchymal Stromal Cells (MSCs) is shown. MSCs in presence of certain growth factors differentiate into multiple lineages by adapting different pathways. Growth factors used are EGF, epidermal growth factors; PDGF, platelet derived growth factor; FGF, Fibroblast growth factor and VEGF, vascular endothelial growth factors. Several pathways mediated that regulation of differentiation are summarized: LPL-lipoprotein lipase gene; ap-2, adipocyte protein; PPAR, peroxisome proliferator activated receptor; BMP-9, Bone morphogenetic protein; TGF, tumor growth factor; GDNF, Glial cell lines derived neurotropic factors; IBMX, isobutyl methyl xanthine.
Whereas decrease in gangliosides biosynthesis contribute towards inhibiting the neuronal differentiation [76]. hMSCs when co-transfected with certain growth factors revealed to maintain the neuronal differentiation, effective in the recovery of hypoxia ischemic brain damage. hMSCs originated from mesodermal line, have potential to transdifferentiate towards the neuronal cells which has revolutionized the regenerative cellular therapy in treatment of neurological disorders [77].
Endodermal lineages: Relatively, it was assumed that hepatocytes could be derived only via endodermal source and the progenitor cells. Fortunately, MSCs have been exposed to hold the ability of transdifferentiation into pancareocytes and hepatocytes upon induction with the growth supplemented media [78]. Human BM derived MSCs were transdifferentiated towards hepatocytes via two steps phases: differentiation phase followed by the maturation phase. Hepatocytes differentiated cells displayed the liver specific transcription factors like nuclear factor 4 α (HNF-4α), albumin and α- fetoprotein. Amongst these factors, HNF-4α is considered to be critical transcription factor for functional and morphological and differentiation to hepatocytes. When human derived MSCs were treated with HNF-4α, it enhanced the differentiation potential of cells and show an increase liver specific expression markers [79]. In another research, it was shown that on extracellular decellularized cell deposited matrix with protein mixture of fibronectin, laminin and collagen along with protein kinases upregulates the activation of hepatic markers [80]. Human dental stem cells are also found to be differentiated towards hepatocytes by induction of the growth factors and particularly melatonin when transplanted during treatment of liver cirrhosis [81]. Moreover, the paracrine factors enhance the differentiation potential and maturation of human BM-MSCs towards the pancreatic lineages without any kind of genetic manipulation and thus transplantation is being used for treating diabetes. Up till now, hMSCs derived from amnion, dental, adipose, umbilical cord, placental tissues and Wharton jelly tissues have effectively differentiated towards insulin forming β-cells. These investigations have shown that hMSCs can differentiate towards the endodermal lineages which can renovate the conventional drug related therapies towards the promising cell based clinical therapies [82,83].
Regulation of differentiation: The commitment as well as differentiation of MSCs from precursors towards specific mature cells is a temporally and tightly controlled method that include the activation of several transcription factors, growth factors, extracellular matrix component and cytokines. Globally, the gene expression profiling with help of DNA microarray technique is rendered as useful tool for identification of genes for commitment and differentiation of stem cells in presence of inductive microenvironments [84]. Several genes are found to have role in differentiation of three lineages of MSCs. One of major intracellular transcription factor include Zinc finger protein ZNF-145 that acts as a upstream regulator of SOX9, thus favoring osteoblastic and adipocytic differentiation of MSCs [85]. The in vitro differentiation analysis of MSCs under proper conditions have progressed in identification of several factors crucial to stem cell commitment process. These factors include secreted molecules along with their receptors like transforming growth factor TGF-β, extracellular matrix components including proteoglycans collagen and actin, interleukin-1 β, nebulette (NEBL) and micro RNAs. Functional investigation of the genes is mandatory to govern how they are tangled in progression and commitment of stem cells towards different stages of differentiation [86-89]. An interesting factor observed during in vitro differentiation is the switching phenomenon of phenotypes among fully differentiated MSC-derived adipocytes, osteoblast and chondrocytes when enthused by stimulus from extra cellular environment.
During this trans differentiation, fully differentiated MSCs undergoes dedifferentiation both in function and morphology and followed by next stimulation, these dedifferentiated cells are re-differentiated into new phenotype. On the whole, it is reasonably concluded that these pre-committed precursor cells along with fully differentiated ones sustain their multipotentiality and that their plasticity during this phenotypic switching phenomenon can be well-maintained under well defined, suitable microenvironmental conditions, observed in a tissue regeneration and repair mechanism [90].
Recent advancement in understanding mechanism of MSCs differentiation involves the members of Wnt family which play their role in osteogenesis of MSCs. Wnts belong to a family of secreted cysteine enriched glycoproteins that have been implied for instructing the stem cell maintenance, propagation, and differentiation in embryonic developmental stages. Canonical Wnt signaling mechanism works as stem cell mitogen by their role in stabilizing intracellular β-catenin receptor and activating the transcription complex, resulting in stimulated expression of cell cycle controlling genes, like cyclin D1, Myc and Msx1. When MSCs are triggered towards Wnt3a, which is a prototypical Wnt signal, under defined growth and medium environment, remarkable increase in cell proliferation along with decrease in the apoptotic process and Wnts mitogenic role in HSCs was observed. Anyhow, overly expressed Wnt3a has led to inhibitory effect on osteogenic differentiation in vitro due to β-catenin arbitrated down regulation of transcription complex. Contrarily, a member of non-canonical Wnt pathway, Wnt5a has been observed to encourage osteogenesis differentiation in vitro. As Wnt3a favors MSC proliferation in early osteogenesis, it is concluded that canonical Wnt signaling pathway has role in the initial stages of osteogenic commitment by enhancing osteoprecursors in stem cell compartment. On the other hand, non-canonical pathway of Wnt regulates the proliferation of these osteoprecursors to from functional mature osteoblasts. Taken into account, Wnt signaling pathway activation leads to a key role in inducing MSCs differentiation. In humans, the mutations in LRP5, a Wnt co-receptor, primed towards the defective bone development. Regain of functional mutation indicates the higher density and mass of bones whereas loss of function results in the complete loss of the bone mass as well as strength, indicating positive feedback effect of Wnt signaling at embryonic osteogenesis stage [12,91].
Characterization of MSCs In-vivo
Current studies have provided appreciated insight for understanding the identity and physiology of bone marrow resident MSCs via novel markers to detect and purify the MSCs enriched cell populations and then assay them in vivo. A platelet derived growth factor receptor, PDGFR, indicates a population of non-hematopoietic cells residing during bone formation process and provides a novel understanding of mechanism involve in MSCs differentiation to adipocytes, reticular cells and osteoblasts, in vivo when transplanted to an recipient [92]. Recently, Nestin, neural stem cell marker, is reported to express in undifferentiated nervous system stem cells. For the first time, expression of the Nestin was checked in progenitor endothelial cells derived from adherent culture of bone marrow cells. Both, BM derived mesenchymal cells and Nestin, can be serially transplanted and used for neovascularization [93] (Figure 3).
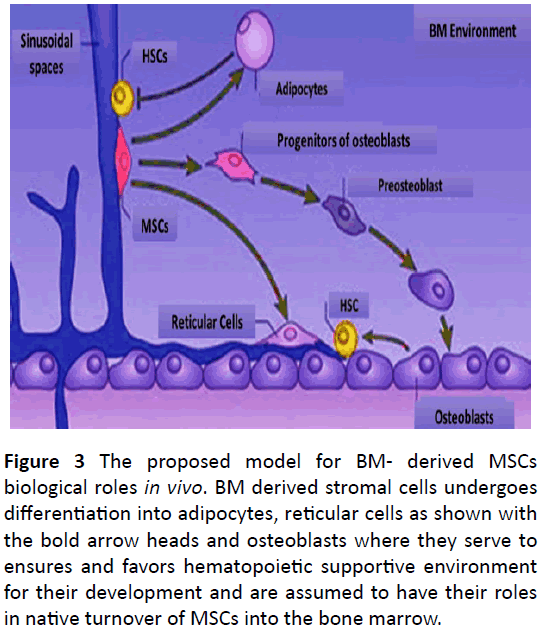
Figure 3: The proposed model for BM- derived MSCs biological roles in vivo. BM derived stromal cells undergoes differentiation into adipocytes, reticular cells as shown with the bold arrow heads and osteoblasts where they serve to ensures and favors hematopoietic supportive environment for their development and are assumed to have their roles in native turnover of MSCs into the bone marrow.
MSCs anatomical analysis in vivo
Researches have influentially explained self-renewing as well as differentiation potential of BM resident mesenchymal stem cells. BM-derived MSCs are being investigated for clinical trials and the innate functions of these cells in BM have been deliberated to know more about the therapeutic role and activity. The location of MSCs has been problematic to trace and even tough to observe vigorously because cavity is not possible to probe easily in vivo and also there is no specific marker for MSC identification in vivo. However, using organ culture system, immunophenotypic analysis, CFU-Fs assays vivo and in vitro labeled assays, MSCs existence in perivascular locations has been supported. This organ culture system has also preserved the original niche of MSCs [94].
Perivascular location has been confirmed by the observations concluding that MSCs are apparently founded in many post-natal organs where their frequency in given tissue is balanced with density of micro-vasculature. MSCs secrete certain angiogenic and trophic factors that contributes in neovasculogenesis along with proliferation and stabilization of endothelial layer depending upon the tissue from which they are derive. The stromal counterparts differentiate and may be migrated towards abluminal sinusoids of marrow and made a three dimensional network which embedded in capillary bed [95].
Acknowledging the capacity of MSCs in forming bone and their assembly into functional bone marrow stroma on heterotopic sites in vivo, a group of researchers recognized the population of osteoprogenitors in the outmost connective tissues layer surrounding BM microvessels. Adventitial reticular cells also known as pericytes projects into sinusoidal lumens with their fibroblastic extensions and are supposed to be in vivo counter parts of CFU-Fs. These pericytes and MSCs share comparable surface and intracellular expressional patterns of proteins which confirms their ontological relation. Moreover, tissue engineering constructs juxtaposed the mesenchymal stromal cells along with endothelial cells and it serves to form long vascularized structures with MSCs naturally exhibiting the pericytic phenotype as well as function. This localization proposes that MSCs may be confidentially involved in angiogenesis, wound healing process and in interacting with blood borne components along with differentiation in multiple lineages into smooth muscle sand myocytes as well [14]. It must be worth to mention that pericytes relates to cells that lies adjacent to capillaries as well as post capillary venules but MSCs are isolated from walls of arteries and veins. Additionally, because pericytes illustrates the wide tissue distribution along microvascular beds and have many functions including phagocytosis, regulating vascular integrity and vessel stabilization. Therefore, regardless of being perivascular, all MSCs cannot be stated as pericytes and vice versa [96].
Fibrocytes represents to be circulating BM-derived cells, found in peripheral blood, are phenotypically similar to monocyte hybrids and fibroblasts articulating the surface marker CD34 and CD35, presence of chemotactic receptors CCR3, CXCR4, CCR5 and CCR7, surface molecules like histocompatibility complex class II MHC-I, MHC-II as well as MSC markers collagen I, collagen 111 and pro-collagen. Moreover, Fibrocytes have also been reported in areas where there was some kind of inflammation, cancer or fibrosis as result of any injury. In these areas, they were supposed to mature towards myofibroblast that reside in tissues. Actually, fibrocytes are found to be phenotypically and functionally similar to BM-MSCs, so recognizing unique characteristics between these two types of cells may help in understanding the MSCs and fibrocytes homology [97].
Role of MSCs in maintenance of hematopoiesis in vivo
As stated, MSCs were initially isolated from bone marrow compartment. Subsequently then, bone marrow derived MSCs have been studied most extensively and are considered to be key managers of bone marrow physiology. Blood cells are constantly produced principally in our bone marrow during adulthood process. MSCs have longer been considered to serve as in vivo progenitors of some non-hematopoietic constituents of BM that control hematopoiesis, like adipocytes, osteoblasts and reticular cells of fibroblasts. Therefore, MSCs are expected to contribute in maintaining homeostasis within hematopoietic compartment in vivo via the regulatory roles of their matured progeny [98].
HSCs are considered to localized in the confined sites within bone marrow microenvironment. This microenvironment is constituted by soluble factors, surrounding cells and extra cellular matrix proteins that latterly contribute in promoting maintenance of HSCs [99]. Osteoblasts have been assumed to significantly contribute towards stem cell niches and promotes regulation of HSCs homeostasis via direct interactions from cell to cell. Though this osteoblast-HSC niche are still debatable, it is believed that osteoblasts serve as vital component in microenvironment of BM either directly or by certain secreting factors by the involvement of signaling pathway and play a key role in developmental stages of hematopoiesis [100]. Additionally, BM stroma also have MSCs derived adipocytes which act to negatively regulate progression of hematopoietic precursors. Molecular mechanism is not known. However several matters concerning the in vivo precise developmental phases of MSCs during differentiation, the pathways leading to the lineage commitment in vivo as well as the ratio of adipocytes and osteoblast production in hematopoietic environment are needed to be considered [101]. Osteoblasts are considered to be significant as niches of HSCs and positively interact and regulate HSCs activity in BM. On the other hand, adipocytes have a role in negative regulation of HSCs activity. CARCs represents CXCL-12 abundant reticular cells that lie adjacent to HSCs as shown in the figure, which have potential to differentiate into adipocytes and osteocytes due their poor characterization and are considered to be originate from BMderived MSCs [3].
Therapeutic Effects of MSCs Related to the Biological Properties
MSCs have the beneficial effects in the medical applications and it requires the complete understanding of their biological properties. There are the four properties of MSCs that play an important role in the medical applications. Firstly, their ability to move to the site of inflammation when given intravenously during the tissue injury. Secondly, their ability to differentiate into different and various cell types. Thirdly, their ability to produce various bio active molecules that are responsible for curing the wounded cells or inhibit the inflammations during the course of injury or any foreign particle invasion. Lastly, the lack of immunogenicity and their ability to perform various immunomodulatory functions [13].
Capacity to migrate, differentiate and engraft at site of injury
After the regular administration of MSCs, they migrate to the sites of injury or inflammation and provide the local beneficial and functional effect at the local sites or tissues. MSCs move selectively to the sites of the injury irrespective to the tissue under diverse pathological condition and is investigated by many studies. Scientists observed that in the mice that has been challenged to bleomycin, the administration of MSCs result in lowering the inflammation of the lungs tissues during injury by moving to the lungs and acquire a phenotype like the epithelium. We also noticed that in the mice, the transplanted MSCs could move to the muscle tissues that has been injured. Migration of the cells totally depend on the signals that is produced by the cells at the injured sites and it consists of the immune cells or growth factors such as chemokines. Migration of MSCs may also be under the control of these signals and chemokines. It has been seen by the various studies that the migration of MSCs is also depend on the growth factors such as receptors of Tyrosine Kinases Growth Factors, Platelets Derived Growth Factors (PDGF), Insulin Growth Factors 1 (IGF1) and several other chemokines [102]. In a study, it has been noticed that the MSCs when injected into mice that has been injured after irradiation, MSCs differentiate into functional cells of the lungs, these functional cells are the epithelial or endothelial cells. Other studies demonstrated that when MSCs is given to the model of the animals that has been injured by exposure of bleomycin, it moves to the lungs and differentiate into epithelial cells or pneumocytes and sustain the phenotype of all the lungs cells such as myofibroblasts, fibroblasts and epithelial cells. Additionally, MSCs could also have the ability to differentiated into the cells of the ectoderm. For example, MSCs when inserted into the Central Nervous Systems (CNS) of the infant mice, it acquired the functional, structural or phenotypic uniqueness of the astrocytes or the neurons. By observing all the facts that MSCs have the ability to differentiate into specialized cells/tissues giving us the opportunity to use the MSCs for the disease treatment [103]. In a model of mouse of Ischemic/Reperfusion (I/R) of the kidney, when MSCs were transplanted at the initial stage of injury, they differentiated into the renal tubular epithelium. The donor cells that are differentiated replaced the empty space that is left over by the dead cells and fill the gap, and hence maintaining the structural features or the tissue and cell repair processes. On the other hand, the clinical aspects of MSCs give us the knowledge to repair the damaged cells by specific differentiation procedures [104].
Releasing the multiple bio active molecules
Clinical applications of MSCs showed that it could release multiple bio active molecules that has the functional or beneficial effects on the cellular dynamics. They include many growth factors such as cytokines and chemokines. Administration of these bio active molecules showed the beneficial effect for tissue and cell repair. For example, in MSCs Conditioning Medium (MSC CM), MSCs move toward the injury sites of the liver and inhibit the liver cells death and regenerate the damaged cells and provide a new department to treat the hepatic failure or liver cirrhosis. Many bio active molecules including the cytokines also takes part in improving the functions of the damaged heart by preventing the apoptosis of the cardiomyocytes and restoring the contractile capacity of the heart and also induce the productive and curative angiogenesis of the damaged or infracted heart. Many of the observed proteins that are secreted as soluble factors are recognized as growth factors such as cytokines and the chemokines. All these molecules have the well-recognized anti apoptotic or the regenerating properties. These properties can be direct or indirect or both. Direct are in producing or activating the signaling intracellularly or indirect by allowing another cell in the small environment to release functionally active bio molecules [105] (Figure 4).
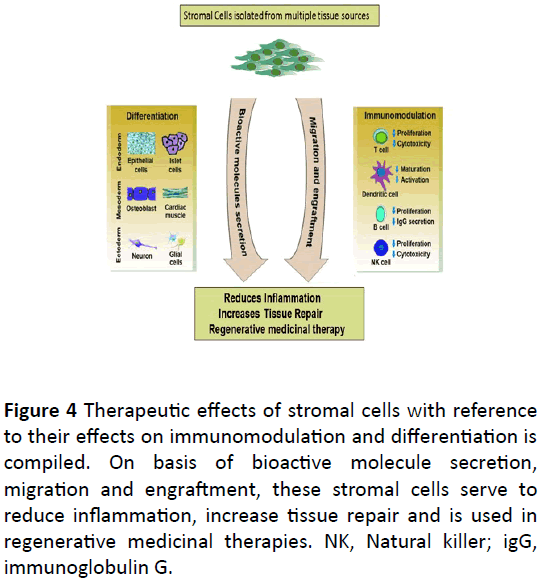
Figure 4: Therapeutic effects of stromal cells with reference to their effects on immunomodulation and differentiation is compiled. On basis of bioactive molecule secretion, migration and engraftment, these stromal cells serve to reduce inflammation, increase tissue repair and is used in regenerative medicinal therapies. NK, Natural killer; igG, immunoglobulin G.
Immunomodulatory aspects of MSCs
MSCs have the distinctive immunologic or immunomodulatory properties that permit their perseverance in the xenogeneic environment. Emerging techniques or data complete or enhance the immunomodulatory functions of the MSCs but the mechanisms of their fundamental immunogenic effects are not yet fully understood by the researchers. Direct connection between the cells or the release of soluble immune suppressive factors or molecules may have a major role in the treatment. MSCs have the ability to communicate with a huge no. of immune cells, that consist of T lymphocytes, B lymphocytes, natural killers and the dendritic cells, the immunomodulatory role of MSCs are mentioned in the Table 1. The immunogenic effects of MSCs are also observed in a variety of animals of the immune diseases as in rat model of transplantation of heart, a long-term Allograft Acceptance is produced by the donor derived MSCs. The immunomodulatory properties of MSCs could also played an important role in the immune disorder, graft versus host diseases (GVHD) treatment [106].
| Immune Cells |
Effects of MSCs on immune Cells |
| T lymphocytes |
- Repress the proliferation of T cells caused by nonspecific mutagenic signals
|
- Enhance the functions of T cells
|
- Adjust and modify the secretion of cytokines of new and Effector
|
|
|
| B lymphocytes |
- Slow down the propagation of B lymphocyte
|
- Affect the chemical characteristics of B cells
|
- Suppress the terminal B-cells differentiation
|
| NK |
- Affect the physical appearance of Natural Killer cells
|
- Inhibit the propagation, secretion of cytokines, and cytotoxic effects
|
| DCs |
- Induce segregation, maturation and the functions of dendritic cells
|
- Inhibit the migration of dendritic cells (DCs) and maturation of antigen presenting cells
|
- Stimulate the mature DCs into the regulatory production of DCs
|
Table 1: Immune-modulatory functions of Mesenchymal Stem Cells (MSCs) effecting different types of immune cells. MSCs are shown to affect activity of immune cells including natural killers NK, dendritic cells, DCs; B lymphocytes and T lymphocytes.
Table 1 shows the immuno-modulatory functions of Mesenchymal Stem Cells (MSCs) effecting different types of immune cells.
Applications of MSCS in gene therapy
The multipotent potential of MSCs and the ability of selfrenewal have shown the promising effect in great number of animal transplantation studies and represented the widespread applications in cell and gene therapy. MSCs that were extended in vitro were responsible for differentiation into cells of the residing tissue, reestablishes its normal function and also renovate the damaged tissue due to trauma or any other disorders. They are not only responsible to repair the tissues of mesenchymal lineages, such as cartilage, bone, cardiomyocytes, and articular cartilage at knee joints, but also repair the cells of other embryonic layers, such as neurons and epithelia in the skin, liver, lung, intestine, spleen, and kidney. These applications express the effectiveness and the plasticity of these adult stem cells in multiple tissue repair and developmental procedures and in the cell therapy applications. It is also notable that MSCs proves to be a principle carrier in delivering the genes into the cell of interest or it does not bring any immune reactivity in the host due to the transplantation. Various strategies have been examined and used to transfer the exogenous DNA into MSCs and make them more useful in tissue regeneration therapies and applications.
Table 1 Immune-modulatory functions of Mesenchymal Stem Cells (MSCs) effecting different types of immune cells. MSCs are shown to affect activity of immune cells including natural killers NK, dendritic cells, DCs; B lymphocytes and T lymphocytes.
Viral transduction, mostly using the adenovirus mediated gene transfer, can produce stable cell clones with high efficacy and the low cell mortality, making it an accepted option in gene therapy applications. On the other hand, the safety concerns related with viral transduction have provoked us to look for another non-viral gene delivery strategies. For instance, the brittle bone disease known as osteogenesis imperfecta is treated by using the MSCs that regenerate the bones in the infected individuals. However, Conventional transfection methods, including calcium phosphate precipitation method, lipofection, and electroporation in delivering the plasmid DNA into MSCs have revealed the little success and result in less than 1% efficiency of transfection method and high cell mortality, so these methods are not useful and appropriate for producing adequate amount of transfected cells for the gene delivery and transplantation [107].
Discussion and Conclusion
It is not easy to isolate the hMSCs but it has been seen that MSC also have the ability of expansion without losing its particular characteristics. On the other hand, besides the mesodermal lineages, MSCs have the ability to differentiate into the endodermal or ectodermal lineages. Moreover, it has been observed that hMSCs also show the immunomodulatory properties by releasing the receptors relevant to immune system and the cytokines that help in the modification of immune system of the host. These distinctive properties of MSCs make them unique from the other stem cells and can be used in the future for cell replacement therapies. Many clinical or preclinical studies demonstrated the usefulness of hMSCs in the treatment of the chronic diseases, such as neurodegenerative diseases, cardiovascular diseases or autoimmune diseases, but on the large experimental or clinical scale there are many questions that must be answer before we use the hMSC. Firstly, the issues of safety of MSCs must be resolved, because when we administered the MSCs for the long-term culturing possible side effects can be seen and it leads to the growth of tumor or metastasis. The second issue is of quality control, additional tests have to be performed before the direct transplantation of MSCs in vivo, these tests include the oncogenic tests, endotoxins assays or the cell viability. An optimum dose has to be decided depending on the disease severity. The third issue is of the clinical production of the MSCs as cells are needed in large number for the clinical utilization of MSCs, in this the in vitro expansion plays a vital role. By this it is concluded that the MSCs obtained from the adult tissues are the favorite choice for the scientists and the complete understanding of the MSCs is necessary to regulate the role of MSCs in the clinical applications.
Current discoveries in the MSCs engineering made it a principle source in the regenerative medicine for the future cell therapies. The clinical experiments of MSCs is now on the way to victory after doing large no. of preclinical experiments, but the complete understanding of mechanism of MSCs is on the preliminary state. The main focus of the future research of MSCs is to isolate the particular markers that is specific for MSCs and also involved the understanding of mechanism of differentiation to revolutionized the therapy of cell regeneration. Special attention should be put on the genetic safety to lower the risk of oncogenesis during cell preparations. However, there should be a dynamic research that focus on the bio banking to develop the novel protocol without disturbing the properties of MSCs in the future on the large scale.
References
- Manfrini M (2012) Human mesenchymal stem cells as a model of study for new biomaterials in bone tissue regeneration. Università degli studi di ferrara.
- Phinney DG (2012) Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J cell biochem 113: 2806-2812.
- Nombela-Arrieta C, Ritz J, Silberstein LE (2011) The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12: 126-131.
- Mitrano TI, Grob MS,Carrión F, Nova-Lamperti E, Luz PA, et al. (2010) Culture and characterization of mesenchymal stem cells from human gingival tissue. J periodontol 81: 917-925.
- Vater C, Kasten P, Stiehler M (2011) Culture media for the differentiation of mesenchymal stromal cells. Acta biomaterialia 7: 463-477.
- Goh TKP, Zhang ZY, Chen AKL, Reuveny S, Choolani M, et al. (2013) Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores open access 2: 84-97.
- Al-Nbaheen M, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al.(2013) Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev Rep 9: 32-43.
- Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, et al. (2014) Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis 5: e1186.
- Ghorbani A, Jalali SA, Varedi M (2014) Isolation of adipose tissue mesenchymal stem cells without tissue destruction: A non-enzymatic method. Tissue and Cell 46: 54-58.
- Diogo MM, Da Silva CL, Cabral JM (2014) Separation technologies for stem cell bioprocessing. Stem Cells and Cell Therapy (1st edn) 157-181.
- Huang J, Zhao L, Xing L, Chen D (2010) MicroRNA?204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem cells 28: 357-364.
- Visweswaran M, Pohl S, Arfuso F, Newsholme P, Dilley R, et al. (2015) Multi-lineage differentiation of mesenchymal stem cells- To Wnt, or not Wnt. Int J Biochem Cell Biol 68: 139-147.
- Salem HK, Thiemermann C (2010) Mesenchymal stromal cells: Current understanding and clinical status. Stem cells 28: 585-596.
- Corselli M, Chen CW, Crisan M, Lazzari L, Péault B (2010) Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 30: 1104-1109.
- Augello A, Kurth TB, De Bari C (2010) Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20: e33.
- Tamama K, Kawasaki H, Wells A (2010) Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. Biomed Res Int.
- Cohnheim J (1867) Uber Entzundung und Eiterung [About inflammation and sepsis]. Pathol Anat Physiol Klin Med 40: 79.
- Maximow AA (1924)Relation of blood cells to connective tissues and endothelium. Physiol rev 4: 533-563.
- Friedenstein A, Piatetzky-Shapiro I, Petrakova K (1966) Osteogenesis in transplants of bone marrow cells. Development 16: 381-390.
- Friedenstein A, Chailakhyan RK, Latsinik NV, AF Panasyuk, Keiliss-Borok IV, (1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplant 17: 331-340.
- Friedenstein AJ, Gorskaja J, Kulagina N (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp hematol4: 267-274.
- Owen M (1988) Marrow stromal stem cells. J Cell Sci 10: 63-76.
- Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9: 641-650.
- Haynesworth S, Goshima J, Goldberg V, Caplan A (1992) Characterization of cells with osteogenic potential from human marrow. Bone 13: 81-88.
- Goshima J, Goldberg VM, Caplan AI (1991) The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate ceramic blocks. Clin Orthop Relat Res 262: 298-311.
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71-74.
- Lazarus H, Haynesworth S, Gerson S, Rosenthal N, Caplan A (1995) Ex-vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone marrow transplant 16: 557-564.
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, et al. (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol18: 307.
- Horwitz EM, Gordon PL, Koo WKK, Marx JC, Neel MD, et al. (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA 99: 8932-8937.
- Koc O, Day J, Nieder M, Gerson S, Lazarus H, et al. (2002) Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone marrow transplant 30: 215.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
- Parekkadan B, Van Poll D, Suganuma K, Carter EA, Berthiaume F, et al. (2007) Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PloS one 2: e941.
- Van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, et al. (2008) Mesenchymal stem cell–derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatol47: 1634-1643.
- Phinney DG, Prockop DJ (2007) Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair-current views. Stem cells 25: 2896-2902.
- Kubo H, Shimizu M, Taya Y, Kawamoto T, Michida M, et al. (2009) Identification of mesenchymal stem cell (MSC)?transcription factors by microarray and knockdown analyses, and signature molecule?marked MSC in bone marrow by immunohistochemistry. Genes to cells, 14: 407-424.
- Pricola KL, Kuhn NZ, Haleem?Smith H, Song Y, Tuan RS (2009) Interleukin?6 maintains bone marrow?derived mesenchymal stem cell stemness by an ERK1/2?dependent mechanism. J cellbiochem108: 577-588.
- Von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, et al. (2012) Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30: 1575-1578.
- Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC (2016) Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol 14: 123-146.
- Kagami H, Agata H, Tojo A (2011) Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering: basic science to clinical translation. Int J Biochem Cell Biol 43: 286-289.
- Zeddou M, Briquet A, Relic B, Josse C, Malaise MG, et al. (2010) The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int 34: 693-701.
- Peng J, Wang Y, Zhang L, Zhao B, Zhao Z, et al. (2011) Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull 84: 235-243.
- Murphy S, Atala A (2013) Amniotic fluid and placental membranes: Unexpected sources of highly multipotent cells. Semin Reprod Med 31: 062-068.
- Karaöz E, Do?an BN, Aksoy A, Gacar G, Akyüz S, et al. (2010) Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 133: 95-112.
- Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T (2012) Comparative investigation of the differentiation capability of bone-marrow-and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res 347: 419-427.
- Fu WL, Zhou CY, Yu JK (2014) A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med 42: 592-601.
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, et al. (2010) Menstrual blood cells display stem cell–like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 19: 439-452.
- Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, et al. (2013) Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013: 1-14.
- Huang HI, Chen SK, Ling QD, Chien CC, Liu HT, et al. (2010) Multilineage differentiation potential of fibroblast-like stromal cells derived from human skin. Tissue Eng Part A 16: 1491-1501.
- Pösel C, Möller K, Fröhlich W, Schulz I, Boltze J, et al. (2012) Density gradient centrifugation compromises bone marrow mononuclear cell yield. PloS one 7: e50293.
- Bourzac C, Smith LC, Vincent P, Beauchamp G, Lavoie JP, et al. (2010) Isolation of equine bone marrow-derived mesenchymal stem cells: A comparison between three protocols. Equine Vet J 42: 519-527.
- Fraser JK, Hicok KC, Shanahan R, Zhu M, Miller S, et al. (2014) The Celution® system: Automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care 3: 38-45.
- Mareschi K, Rustichelli D, Calabrese R, Gunetti M, Sanavio F, et al. (2012) Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: A more advantageous method for clinical use. Stem Cells Int 2012: 1-10.
- Yoon JH, Roh EY, Shin S, Jung N, Song EY, et al. (2013) Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. Biomed Res Int 2013: 1-8.
- Rozemuller H, Prins HJ, Naaijkens B, Staal J, Bühring HJ, et al. (2010) Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem Cells and Development 19: 1911-1921.
- Fan G, Wen L, Li M, Li C, Luo B, et al. (2011) Isolation of mouse mesenchymal stem cells with normal ploidy from bone marrows by reducing oxidative stress in combination with extracellular matrix. BMC Cell Bio 12: 30.
- Watchrarat K, Korchunjit W, Buranasinsup S, Taylor J, Ritruechai P, et al. (2017) MEM α promotes cell proliferation and expression of bone marrow derived equine mesenchymal stem cell gene markers but depresses differentiation gene markers. J Equine Vet Sci 50: 8-14.
- Mostafa NZ, Fitzsimmons R, Major PW, Adesida A, Jomha N, et al. (2012) Osteogenic differentiation of human mesenchymal stem cells cultured with dexamethasone, vitamin D3, basic fibroblast growth factor, and bone morphogenetic protein-2. Connect Tissue Res 53: 117-131.
- Oliver-Vila I, Coca MI, Grau-Vorster M, Pujals-Fonts N, Caminal M, et al. (2016) Evaluation of a cell-banking strategy for the production of clinical grade mesenchymal stromal cells from Wharton's jelly. Cytotherapy 18: 25-35.
- Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, et al. (2010) in vitro high?capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem cells 28: 788-798.
- Gharibi B, Hughes FJ (2012) Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med 1: 771-782.
- Menssen A, Häupl T, Sittinger M, Delorme B, Charbord P, et al. (2011) Differential gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenic development. BMC genomics 12: 461.
- Muruganandan S, Parlee SD, Rourke JL, Ernst MC, Goralski KB, et al. (2011) Chemerin, a novel peroxisome proliferator-activated receptor γ (PPARγ) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem 286: 23982-23995.
- Muruganandan S, Roman A, Sinal C (2009) Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell Mol Life Sci 66: 236-253.
- Wang W, Olson D, Cheng B, Guo X, Wang K (2012) Sanguis draconis resin stimulates osteoblast alkaline phosphatase activity and mineralization in MC3T3-E1 cells. J Ethnopharmacol 142: 168-174.
- Zhang Y, Su J, Yu J, Bu X, Ren T, et al. (2011) An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. J Bone Miner Res 26: 604-617.
- Xu D, Zhao Y, Wang J, He J, Weng Y, et al. (2012) p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB reports 45: 247-252.
- Birmingham E, Niebur G, McHugh P (2012) Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater 23: 13-27.
- Wang J, Liao J, Zhang F, Song D, Lu M, et al. (2017) NEL-Like Molecule-1 (Nell1) Is Regulated by bone morphogenetic protein 9 (BMP9) and potentiates BMP9-induced osteogenic differentiation at the expense of adipogenesis in mesenchymal stem cells. Cell Physiol Biochem 41: 484-500.
- Oliveira F, Bellesini L, Defino H, Herrero CS, Beloti M, et al. (2012) Hedgehog signaling and osteoblast gene expression are regulated by purmorphamine in human mesenchymal stem cells. J Cell Biochem 113: 204-208.
- Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, et al. (2012) Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 50: 477-489.
- Liu CF, Lefebvre V (2015) The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res 43: 8183-8203.
- Karl A (2013) Role of BMP and TGFß signalling in the terminal differentiation in the in vitro chondrogenesis of mesenchymal stem cells.
- Statman LY (2012) Mechanosensitive β-Catenin signaling modulates mesenchymal stem cell chondrogenesis, University of California, San Francisco.
- Chen L, He DM, Zhang Y (2009) The differentiation of human placenta-derived mesenchymal stem cells into dopaminergic cells in vitro. Cell Mol Biol Lett 14: 528-536.
- Yu YL, Chou RH, Chen LT, Shyu WC, Hsieh SC, et al. (2011) EZH2 regulates neuronal differentiation of mesenchymal stem cells through PIP5K1C-dependent calcium signaling. J Biol Chem 286: 9657-9667.
- Yang HJ, Jung KY, Kwak DH, Lee SH, Ryu JS, et al. (2011) Inhibition of ganglioside GD1a synthesis suppresses the differentiation of human mesenchymal stem cells into osteoblasts. Dev Growth Differ 53: 323-332.
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, et al. (2011) Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134: 1777-1789.
- Ayatollahi M, Soleimani M, Tabei SZ, Kabir Salmani M (2011) Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J Stem Cells 3: 113-121.
- Bishi DK, Mathapati S, Venugopal JR, Guhathakurta S, Cherian KM, et al. (2013) Trans-differentiation of human mesenchymal stem cells generates functional hepatospheres on poly (L-lactic acid)-co-poly (ε-caprolactone)/collagen nanofibrous scaffolds. J Mater Chem B 1: 3972-3984.
- He H, Liu X, Peng L, Gao Z, Ye Y, et al. (2013) Promotion of hepatic differentiation of bone marrow mesenchymal stem cells on decellularized cell-deposited extracellular matrix. BioMed Res Int 2013: 406871.
- Cho YA, Noh K, Jue SS, Lee SY, Kim EC (2015) Melatonin promotes hepatic differentiation of human dental pulp stem cells: clinical implications for the prevention of liver fibrosis. J Pineal Res 58: 127-135.
- Prabakar KR, Domínguez-Bendala J, Molano RD, Pileggi AS, Ricordi VC (2012) Generation of Glucose-Responsive, Insulin-Producing Cells From Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Cell Transplant 21: 1321-1339.
- Cao X, Han ZB, Zhao H, Liu Q (2014) Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int J Biochem Cell Biol 53: 372-379.
- Hsiao STF, Asgari A, Lokmic Z, Sinclair R, Dusting GJ (2011) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Develop 21: 2189-2203.
- Liu TM, Guo XM, Tan HS, Hui JH, Lim B (2011) Zinc?finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheumatol 63: 2711-2720.
- Sonomoto K, Yamaoka K, Oshita K, Fukuyo S, Zhang X (2012) Interleukin?1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt?5a/receptor tyrosine kinase–like orphan receptor 2 pathway. Arthritis Rheumatol 64: 3355-3363.
- Aino M, Nishida E, Fujieda Y, Orimoto A, Mitani A, et al. (2014) Isolation and characterization of the human immature osteoblast culture system from the alveolar bones of aged donors for bone regeneration therapy. Expert Opin Biol Ther 14: 1731-1744.
- Ragni E, Montemurro T, Montelatici E, Lavazza C, Viganò M, et al. (2013) Differential microRNA signature of human mesenchymal stem cells from different sources reveals an “environmental-niche memory” for bone marrow stem cells. Exp Cell Res 319: 1562-1574.
- Park JS, Chu JS, Tsou AD, Diop RZ, Wang TA, et al. (2011) The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 32: 3921-3930.
- Ullah M, Sittinger M, Ringe J (2014) Transdifferentiation of adipogenically differentiated cells into osteogenically or chondrogenically differentiated cells: Phenotype switching via dedifferentiation. Int J Biochem Cell Biol 46: 124-137.
- Baksh D, Boland GM, Tuan RS (2007) Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem 101: 1109-1124.
- Caplan AI, Correa D (2011) PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res 29: 1795-1803.
- Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H (2010) The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem. Cytochem 58: 721-730.
- Choi MY, Kim HI, Yang YI, Kim JT, Jang SH, et al. (2012) The isolation and in situ identification of MSCs residing in loose connective tissues using a niche-preserving organ culture system. Biomaterials 33: 4469-4479.
- Hoch AI, Binder BY, Genetos DC, Leach JK (2012) Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PloS one 7: e35579.
- Corselli M, Chen CW, Sun B, Yap S, Rubin JP, (2011) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21: 1299-1308.
- Fu Xy, Zhang Dw, Li Yd, Zhao Pw, Tang YQ (2015) Curcumin treatment suppresses CCR7 expression and the differentiation and migration of human circulating fibrocytes. Cell Physiol Biochem 35: 489-498.
- Pontikoglou C, Deschaseaux F, Sensebé L, Papadaki HA (2011) Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev 7: 569-589.
- Nombela-Arrieta G, Pivarnik B, Winkel KJ, Canty B, Harley JE, et al. (2013) Quantitative imaging of hematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 15: 533-543.
- Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, et al. (2012) The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149: 63-74.
- Bethel M, Chitteti BR, Srour EF, Kacena MA (2013) The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Curr Osteoporos Rep 11: 99-106.
- Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D (2011) Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res 2011: 207326.
- Bhaskar B, Mekala NK, Baadhe RR, Rao PS (2014) Role of signaling pathways in mesenchymal stem cell differentiation. Curr Stem Cell Res Ther 9: 508-512.
- Zou X, Gu D, Xing X, Cheng Z, Gong D, et al. (2016) Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats, Am J Transl Res 8: 4289.
- Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, et al. (2011) Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 6: 206-214.
- De Witte SF, Franquesa M, Baan CC, Hoogduijn MJ (2015) Toward development of iMesenchymal stem cells for immunomodulatory therapy. Front Immunol 6: 648.
- Uchibori R, Tsukahara T, Ohmine K, Ozawa K (2014) Cancer gene therapy using mesenchymal stem cells. Int J Hematol Res 99: 377-382.





