Shaoshan Cui1,2*, Yuanhong Li1, Hongguang Lu1,3, Xing-Hua Gao1, Huachen Wei1,4 and Hong-Duo Chen1*
1Department of Dermatology, No. 1 Hospital of China Medical University, Shenyang 110001, China
2Department of Dermatology, No. 1 Hospital of Dalian Medical University, DaLian, 116011, China
3Department of Dermatology, Affiliated Hospital, Guiyang Medical College, Guiyang 550001, China
4Department of Dermatology, Mount Sinai School of Medicine, New York, NY 10029, USA
*Corresponding Author:
Shaoshan Cui
Department of Dermatology
No. 1 Hospital of China Medical University
Shenyang 110001, China
E-mail: cuishaoshan1225@126.com
Hong-Duo Chen
Department of Dermatology
No. 1 Hospital of China Medical University
Shenyang 110001, China
E-mail: chenhd@cae.cn
Received date: May 04, 2016; Accepted date: May 30, 2016; Published date: June 03, 2016
Citation: Cui S, Li Y, Lu H, et al. Morphological Relationship between Nerve Fibers and Melanocytes in the Epidermis of Melasma. Clin Pediatr Dermatol. 2016, 2:2 Doi: 10.21767/2472-0143.100020
Keywords
Nerve fibers; Melanocytes; Melasma
Introduction
Melanocyte is the melanin-producing cell and is of neural crest origin. Melasma is a common acquired circumscribed hypermelanosis primarily occurring on the faces of women. It is known that the facial hyperpigmentation of melasma patients becomes more prominent when physically exhausted or emotionally distressed [1,2]. The precise feature of the connection between stress and exacerbation of skin inflammation has perplexed researchers. Studies have shown the evidence that stress travels to the skin through peripheral neuropeptidergic nerve fibers and exacerbates the neurogenic inflammatory aspect of dermatoses [3]. Sensory neurons of the peripheral nervous system send many primary afferent fibers to the skin. Melanocytes in human skin are known to have certain morphological links with the nerve fibers [4]. However, quantitative analysis of the morphological relationship between nerve fibers and melanocytes in the lesions of melasma have not been reported, which we delineated in this study.
Materials and Methods
Skin biopsies
All subjects were enrolled into the study from No.1 Hospital of China Medical University, Shenyang, China, upon completion of the informed consent form. Normal control subjects were recruited with the exclusion of systemic and/or dermatological diseases within one year. The melasma patients were enrolled based on the clinical diagnostic criteria and pathological examination. Facial skin biopsies of the normal control subjects, and lesional skin and normal-appearing skin of the opposite side of the melasma patients were collected. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by our Institutional Medical Ethic and Human Research Committee.
Biopsy and specimen processing
Incisional biopsy of 0.5 cm × 0.5 cm specimen embedded immediately in OCT Compound (Sakura Finetek, USA) with liquid nitrogen, and stored at -70? until further processing. Blocks were cryosectioned at a thickness of 12 μm, dried at the room temperature, fixed by cold acetone for 10 min.
Double staining for melanocytes and nerve fibers
The double labeled immunofluorescency was performed as described with a slight modification [5]. Briefly, anti-neurofilament H 200Kd rabbit serum (1:100; Serotec, UK), followed by tetraethyl rhodamine-conjugated goat anti-rabbit IgG (1:100; Southern Biotechnology, USA) was used to stain the cutaneous nerves. Antimelanosome mouse IgG monoclonal antibody (1:5; NKl/beteb, Sanbio B.V., Netherlands), followed by fluoresceinisothiocynate labeled goat anti-mouse IgG (1:100; Serotec, UK) was used to stain melanocytes. The negative controls were performed by replacing the primary antibodies with normal rabbit or mouse serum. All slides were examined under a confocal laser scanning microscope (Leica, Germany). A total of 30 melanocytes were randomly examined. The percentage of green fluorescent melanocytes having contact with red fluorescent fibers was counted and calculated.
Statistical analysis
Statistical analysis was performed using SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA). Non-parametric test (Kruskalwallis H) was performed to analyze the data. A p value<0.05 was considered to be statistically significant.
Results
Total of 31 facial skin biopsies from the normal control subjects were obtained from the clinic of plastic surgery. Biopsies of 36 lesional skin and 31 normal-appearing skin of the opposite side of the melasma patients were collected from the outpatient clinic of dermatology. All the subjects were female. The average age of melasma patients and normal subjects were 28-45 (34.2 ± 9.6) years and 25-50 (33.3 ± 10.4) years, respectively.
About 42.3 ± 5.6% of melanocytes in epidermis of normal control subjects had the intimate contacts with nerve fibers (Figure 1). The morphological contacts of melanocytes with the intraepidermal nerve fibers were significantly increased not only in the melasma lesions (74.5 ± 6.7%), but also in the normalappearing skin of the opposite sites of melasma lesions (69.4 ± 7.3%) (Figure 2). Significant difference was seen between normal control skin and melasma lesion (p<0.05) or between normal control skin and normal-appearing skin of the melasma patients (p<0.05). The difference in the number of neural-melanocytic contacts between the lesional and normal-appearing skin of the melasma patients was also statistically significant (p<0.05).
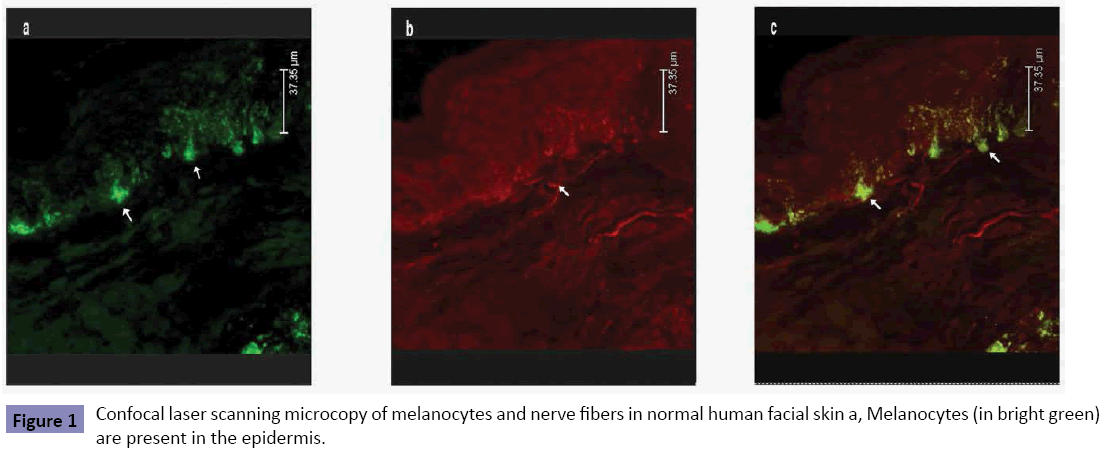
Figure 1: Confocal laser scanning microcopy of melanocytes and nerve fibers in normal human facial skin a, Melanocytes (in bright green) are present in the epidermis.
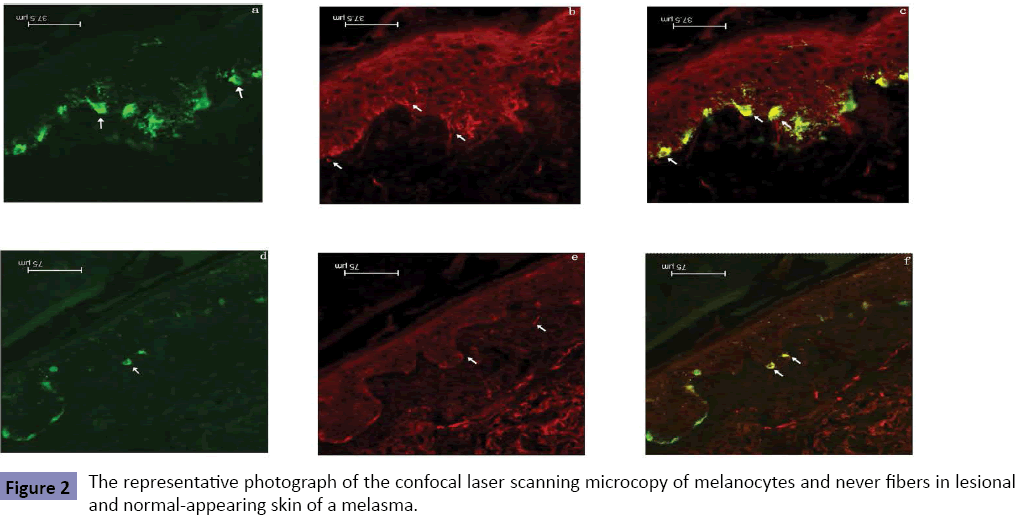
Figure 2: The representative photograph of the confocal laser scanning microcopy of melanocytes and never fibers in lesional and normal-appearing skin of a melasma.
Discussion
Human melanocytes are dendritic cells of neural crest cell origin with monophenolase activity and distinctive melanosomes. The skin melanocytes have a close contact with the surrounding keratinocytes via their dendritic processes. This physical connection enables melanocytes to transfer melanin into keratinocytes to define the skin color and protect against damage from ultraviolet radiation. Several environmental and physiological factors such as ultraviolet radiation and α-melanocyte stimulating hormone are known to regulate the quantity and quality of melanin in the skin of various species [6]. A balance in these regulatory factors is essential for the normal melanocytic development and function. Any derangement that causes a deficiency or over stimulation has an abnormal effect on melanocytic function. It is generally accepted that skin is a target organ of the stress reaction [7-9]. The function of melanocytes are also affected by physical and emotional stresses [10,11].
Melasma is an acquired circumscribed facial hyperpigmentation commonly seen in Asian women. A number of anecdotal clinical observations have shown that when the melasma patients are physically exhausted or emotionally distressed, the facial skin hyperpigmentation becomes more obvious [12-14]. The psychoneural impact on melasma was described as early in 1742 in an ancient textbook of Chinese traditional medicine entitled “Golden Mirror for Original Medicine-Surgical Secrets”. It says that “Dark dyschromia comes to face due to the blood deficiency and malnutrition resulting from anxiety and depression”. Anecdotal reports related the emotional changes, depression, athymia and neurastheria to the facial discoloration. It has demonstrated close interactions between the nervous and immune systems that regulate peripheral inflammation and link psychosocial stress with chronic somatic disease [12,13].
Although the biological significance of the intimate contacts between the nerve fibers and melanocytes remains speculative, our finding provides evidence that neural system may directly regulate certain biological functions of melanocytes. Moreover, how the morphological contact between the nerve fibers and melanocytes contributes to the skin pigmentation remains unclear and needs further research.
As is well known, the skin color of certain species of frogs and fish changes with surroundings, which has been found to be relevant to the excitation of sympathetic nerve and/or parasympathetic nerve. The color changes of human skin are also related to autonomic nerve. The inhibitory factors generated while sympathetic nerve is excessively active have rivalry effects on α-melanocyte stimulating hormone and reduce pigment, while parasympathetic nerve can deepen pigment. It is generally thought that onset of vitiligo is a kind of disorder of immune function, psychological effects and endocrine secretion under the stimulus of various factors [10,15].
In the skin, nerve fibers may secrete neuromediators, such as calcitonin-gene related peptide, substance P, vasoactive intestinal peptide, and α-melanocyte stimulating hormone. Neuromediators are also found to be expressed in the skin to modulate the functions of skin cells [16,17]. The nerve system, endocrine system, and cutaneous immune system are not independent respectively but closely associated and interact with each other via the same language of cytokines and neurotransmitters.
Crosstalk between the skin and its microbial environment is highlighted and they cooperate to maintain the homeostasis of the skin via the so-called neuro-immuno-cutaneous network. These neuromediators cooperate to maintain the homeostasis of the skin, via the so-called neuro-immuno-cutaneous network [18]. A number of common dermatologic diseases have some form of psychomediated pathogenesis that partially explains the development of skin lesions. There is a link between emotional stressors (acute or chronic), psychiatric diseases, and dermatoses. Our study provides a direct evidence for the morphological links of this network.
At present the creature-psychology-community medicine formula has gradually become the main medical trend, and psychological elements have been receiving increasing attention. Nerve system and skin are closely linked anatomically and physiologically. Psychological elements may affect the flare-up and severity of skin diseases via the neuro-immuno-cutaneous-endocrine network. Therefore, the further investigation of the neuroimmuno- cutaneous-endocrine network will provide new insights into etiologies of and the diagnosis and therapeutic modalities for dermatogical diseases. Clarification of the role of this alternative stress axis may enable the design of novel therapeutic strategies [9].
In summary, our study provides morphological evidence of the contacts between intraepidermal nerve fibers and melanocytes in melasma. The neural-melanocytic contacts in the lesional skin of the melasma patients were statistically more than those in the normal-appearing skin of the opposite side of the same individuals and those in normal controls. Our finding also provides evidence that the skin color is linked to the central nervous system via the peripheral nerve fibers in the skin, corroborating the proverbs “The skin is the mirror which reflects the state of the mind” and “The skin is the window of the mind”.
Conflict of Interest
The authors state no conflict of interest.
Acknowledgements
This work was supported partly by the programs of Chang Jiang Scholars and Innovative Teams in Universities (IRT0640), Ministry of Education, China.
References
- Handel AC, Lima PB, Tonolli VM, Miot LD, Miot HA (2014) Risk factors for facial melasma in women: a case-control study. Br J Dermatol 171: 588-594.
- Rigopoulos D, Gregoriou S, Katsambas A (2007) Hyperpigmentation and melasma. J Cosmet Dermatol 6: 195-202.
- Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, et al. (2008) Further Exploring the Brain-Skin Connection: Stress Worsens Dermatitis via Substance P-dependent Neurogenic Inflammation in Mice. J Invest Dermatol 128: 434-446.
- Hara M, Toyoda M, Yaar M, Bhawan J, Avila EM, et al. (1996) Innervation of melanocytes in human skin. J Exp Med 184: 1385-1395.
- Mellgren SI, Nolano M, Sommer C (2013) The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol 15: 171-188.
- Li A (2014) The biology of melanocyte and melanocyte stem cell. Acta Biochim Biophys Sin (Shanghai) 46: 255-260.
- Sarah D (2012) Psychodermatology: An emotional response. Nature 492: S62-63.
- Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R (2006) Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 126: 1697-1704.
- Liborija L, Luka L, Josip M, Vlasta V, Nina T, et al. (2013) Psychoneuroimmunologic Aspects of Skin Diseases. Acta Clin Croat 52: 337-345.
- Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, et al. (2013) Vitiligo: interplay between oxidativestress and immune system. Experimental dermatology 22: 245-250.
- Inoue K, Hosoi J, Ideta R, Ohta N, Ifuku O, et al. (2003) Stress-augmented ultraviolet-irradiation-induced pigmentation. J Invest Dermatol 121: 165-171.
- Handel AC, Miot LD, Miot HA (2014) Melasma: a clinical and epidemiological review. An Bras Dermatol 89: 771-782.
- Seçkin HY, Kalkan G, Bas Y, Akbas A, Önder Y, et al. (2014) Oxidative stress status in patients with melasma. Cutaneous and Ocular Toxicology 33: 212-217.
- Harumi O, Goh CL (2016) The Effect of Melasma on the Quality of Life in a Sample of Women Living in Singapore. J Clin Aesthet Dermatol 9: 21-24.
- Angelo P, Damiano A (2001) Stressful Life Events and Skin Diseases: Disentangling Evidence from Myth. Psychother Psychosom 70: 118-136.
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M (2006) Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86: 1309-1379.
- Ashrafi M, Baguneid M, Bayat A (2015) The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm Venereol.
- Brazzini B, Ghersetich I, Hercogova J, Lotti T (2003) The neuro-immuno-cutaneous network:relationship between mind and skin. Dermat ol Ther 16: 123-131.