Research Article - (2023) Volume 14, Issue 2
Received: 27-Sep-2022, Manuscript No. AASRFC-22-14440; Editor assigned: 30-Sep-2022, Pre QC No. AASRFC-22-14440 (PQ); Reviewed: 14-Oct-2022, QC No. AASRFC-22-14440 ; Revised: 21-Feb-2023, Manuscript No. AASRFC-22-14440 (R); Published: 28-Feb-2023, DOI: 10.36648/0976-8610.14.2.13
Purpose: The lack of a uniform Minimum Data Set (MDS) in Iran for recording and reporting the Hematopoietic Stem Cell Transplantation (HSCT) recipients across all treatment processes leads to determining a localized MDS by considering two well-known international MDS. This study describes the required MDS development for the hematopoietic stem cell transplantation recipient registry.
Method: The study was conducted in 2020-2021. An expert panel of three hematology and oncology specialists and three medical informatics were formed. After reviewing data sets of HSCT registries, focus groups, meetings, questionnaire development and validation, an MDS was determined.
Results: The validated MDS comprises 1177 data elements in 9 sections, including pre-HSCT, leukemia, lymphoma, myelodysplastic syndrome, myeloproliferative neoplasms, combined myelodysplastic syndrome and myeloproliferative neoplasms, plasma cell disorders, hemoglobinopathy and post- HSCT. The data elements were aggregated by the expert panel based on the MDS of European Bone Marrow Transplantation (EBMT) and Center for International Blood and Marrow Transplant Research (CIBMTR) registries and the medical records of the transplantation center of Imam Khomeini hospital complex. 399 data items of 1576 were rejected according to the expert's opinion.
Conclusion: In the present study, the validated MDS were developed and will be used in the registry system in the future to record and report the uniform data items of HSCT-related data.
Essential data set; Minimum data set; Registry; Hematopoietic stem cell transplantation; HSCT
Hematopoietic Stem Cell Transplantation (HSCT) is a curative procedure for malignant and non-malignant hematologic diseases [1]. In Iran 11,000 hematopoietic stem cell transplants have been performed annually and this number is increasing. One of the HSCT centers in Iran is the HSCT center of the Imam Khomeini hospital complex, affiliated with the Tehran university of medical sciences. This center has been operating since 2007 and has performed more than 650 transplants. Hematopoietic stem cell transplantation procedure in some diseases is a well-known treatment used. In others, it is almost a new treatment that needs to be further investigated and observed. Recording and reviewing transplant outcomes according to patients characteristics, transplant conditions and other factors can help better understand this treatment's details and improve and modify it. The transplant procedure is complex and cost-intensive. It needs to reduce complications and mortality rates, improve transplantation survival rates and increase the quality of recipients' lives. There are some ambiguities regarding stem cell transplantation procedures, the factors that affect transplant success and the factors that contribute to mortality and post-transplant diseases. To respond to these recent questions, higher-quality databases are required. Therefore, the availability and accessibility of proper datasets and information related to the transplant procedure, recipient and donor characteristics are needed. The appropriate tool for managing transplant data is an outcome registration system. The first purpose of the registry is to monitor outcomes and report the procedure details and the quality of care. The further objective is to improve the procedure and conditions for transplants and increase the quality of care and life. The fundamental step in designing an efficient registry is determining the minimum data elements. A Minimum Data Set (MDS) is the least amount of essential data elements among the defined HSCT related data elements. Many studies have emphasized the importance of a minimum dataset in creating the registry and its impact on registering and managing valuable and necessary information. One of the most critical factors for the success or failure of a registry system is the selection of appropriate data items [2-5]. Although there have been reports on determining the minimum data elements for some hematologic diseases and malignancies in Iran [6,7], none have focused on HSCT. Therefore, this study focuses on utilizing this procedure in hematologic disorders and malignancies treatment. The current study is a comprehensive study on the registration data items of HSCT in Iran for seven diseases with considering the data related to the recipient, donor and transplantation details and follow-up information for the recipient. Data collection based on standard and uniform MDS earns efficient and complete information required for outcome assessment and research. This may further help the transplant research center collaborate with other transplant centers globally to improve the treatment procedure and care details [8]. However, based on the research done, no study has been performed to identify HSCT-MDS or design an HSCT registry in Iran. Therefore, this study was conducted to determine an MDS as a standard tool in further HSCT registry development process in the Imam Khomeini Hospital complex to collect, analyze and report HSCT information.
The study objective was to develop a Minimum Data Set (MDS) for the HSCT recipient's registration system. It is designed in three phases, including literature review, focus group for requirement analysis and questionnaire development for data validation, explained in the following sections. The current research has formal ethical approval for the design, development and deployment of the registry and using the transplant data of the HSCT center of Imam Khomeini hospital complex of Tehran university of medical sciences (IR.TUMS.SPH.REC.1399.043). This descriptive crosssectional study was conducted in 2020-2021.
Phase 1: Literature Review
First, a literature review was performed to identify available MDS in HSCT-recipient registries worldwide. So, the scientific databases, including PubMed, Scopus and Embase, were investigated to retrieve related sources. The phases of data collection and selection based on eligibility criteria are presented in the PRISMA flow diagram (Figure 1). Moreover, a search was done in the Google search engine to find material related to registries and MDS of HSCT, which were retrieved from databases (Figure 1).
Figure 1: PRISMA flow diagram of the study selection process.
The search strategy was created according to two significant concepts: the "HSCT" and the "minimum dataset and registry" without date restriction. The search was performed in Embase, PubMed and Scopus databases. The Google search engine searched the publications and websites related to HSCT registries. The resources, including original work, reports, texts, websites, proceedings, abstracts and reviews matching the search query and related explicitly to the HSCT recipient's registry and MDS in english, were included. The Studies with non-english language, letters to the editor, short communication and articles with no available full text were excluded. This represents the PubMed search query. Other databases were searched with similar queries based on their formats. Among national and international registries retrieved from the search phase, two registries have more comprehensive and detailed datasets and MDS that collaborate with more stem cell transplant centers worldwide: the European society for Blood and Marrow Transplantation (EBMT) registry and the Center for International Blood and Marrow Transplant Research (CIBMTR) registry (Table 1).
| Databases | Embase, PubMed, Scopus |
|---|---|
| #1 | Stem cell transplantation (title/abstract) or stem cell transplant (title/abstract) or autologous (title/abstract) or autograft* (title/abstract) or auto transplant* (title/abstract) or ((cord blood(title/abstract) or hematopoietic (title/abstract) or mesenchymal (title/abstract) or haploidentical (title/abstract)) and (stem cell transplant* (title/abstract)) or homologous (title/abstract) or ((allogeneic (title/abstract)) and (graft* (title/abstract) or transplant* (title/abstract)) or allograft* (title/abstract) or homograft* (title/abstract) or HSCT (title/abstract) or AHSCT (title/abstract) or bone marrow (title/abstract) or BMT (title/abstract) or GVHD (title/abstract) or Graft vs. host disease (title/abstract) |
| #2 | Registry (title/abstract) or register* (title/abstract) or minimum dataset (title/abstract) |
| Search | #1 and #2 |
| Limit | language=english, species studies=humans |
Table 1: The search strategy for PubMed database.
203 of 740 records were read as abstracts. The related records were studied in full texts (158 records) and the HSCT recipient registry information was extracted. Next, a general search to find more national and international registries was done using the Google search engine. After that, each registry was searched and its website was considered for more information and MDS of the registry.
Phase 2: Requirement Analysis and Focus Group
The expert panel included three hematology and oncology specialists of the HSCT center of Imam Khomeini hospital complex and three medical informatics experts from the health information management and medical informatics department of Tehran University of medical sciences. The characteristics of experts' panel presented. In the meetings held with the experts' panel, first, the experts identified the diseases for which the transplant is performed in this center.
Second, they discussed the similar HSCT registration systems retrieved from the search phase. In order to select similar registries, the national and international registries were surveyed by experts. However, because the national registries were designed based on the needs of the target population of that country, national registration systems were discarded. Therefore, according to experts' opinions, only international registries were considered. Data items were collected from the global registries or added based on the experts' ideas if not in the dataset. Among international registries retrieved from the search phase, experts unanimously agreed with the two registries that collaborate with more stem cell transplant centers worldwide: the European society for Blood and Marrow Transplantation (EBMT) registry and the Center for International Blood and Marrow Transplant Research (CIBMTR) registry (Table 2).
| Experts | Number | Gender | Education degree |
|---|---|---|---|
| Hematology and oncology specialists | 3 | Male: 3 Female: 0 | Fellowship |
| Medical informatics | 3 | Male:1 Female:2 | PhD |
Table 2: Experts' panel characteristics.
Phase 3: Questionnaire Development for Data Validation
In the first step, the data elements collected from the two selected registries were investigated and aggregated. Based on the aggregated data items, an MDS checklist was created. Then, a focus group that included three hematology oncology specialists with a minimum of five histories of HSCT was held. The focus group aims to deliberate the main categories of necessary data elements. Moreover, individuals discussed the checklist and the points were noted to apply. After two sessions, the first draft of the MDS questionnaire was designed. The second step examined the questionnaire's content validity and reliability. The questionnaire's content validity was evaluated qualitatively by an expert panel including five hematology and oncology experts for clinical data items and five medical informatics experts for demographic data items. Table 3 present the expert panel characteristics. The questionnaire was evaluated by experts and modified based on their feedback. Cronbach's alpha examined the reliability of the questionnaire. The Cronbach's alpha is a measure of internal consistency to earn the MDS questionnaire's homogeneity. The analysis of questionnaire reliability was performed in IBM SPSS statistics version 26 [9]. The Cronbach's alpha value for the entire MDS questionnaire was 0.84, which means the reliability of the MDS questionnaire was good. The Cronbach's alpha value for each category is presented in the Table 4.
| Experts | Number | Gender | Age group | Education degree |
|---|---|---|---|---|
| Hematologists | 5 | Male: 4 Female: 1 | 38-60 | specialty |
| Medical informatics | 5 | Male: 1 Female: 4 | 45-65 | PhD |
Table 3: Experts panel characteristics.
| No. | Category | Cronbach's alpha value | Reliability level |
|---|---|---|---|
| 1 | Pre-HSCT | 0.73 | Acceptable |
| 2 | Leukemia | 0.76 | Acceptable |
| 3 | Lymphoma | 0.83 | Good |
| 4 | Myelodysplastic syndrome | 0.88 | Good |
| 5 | Myeloproliferative neoplasms | 0.87 | Good |
| 6 | Myelodysplastic syndrome/myeloproliferative neoplasms | 0.87 | Good |
| 7 | Plasma cell disorders including multiple myeloma | 0.93 | Excellent |
| 8 | Hemoglobinopathy | 0.83 | Good |
| 9 | Post-HSCT | 0.83 | Good |
Table 4: Cronbach's alpha value of each category (questionnaire).
Phase 4: Defining the Final Version of the MDS of HSCT
The questionnaire Content Validity was examined quantitatively by the Content Validity Ratio (CVR). It is utilized to determine the necessary data elements by expert panels. The questionnaire was presented to the expert panel in a table template with three columns' essential', 'useful, but not essential or impossible to do and not essential for each data item to determine the essentiality of the items. The CVR was calculated based on the following formula for each data item.
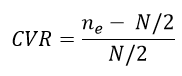
"ne" is the number of experts indicating the 'essential' option for an element and 'N' is the total number of experts [10,11]. Finally, the value of each data item considering the number of experts compared with the values in Lawshe's table [12-14]. In the current study, the number of experts was five.
Accordingly, each data element with a value equal to or higher than the value of Lawshe's table was preserved in the registry dataset; otherwise, it should be removed from the data items list. Therefore, based on Lawshe's table, with five experts, the threshold of necessary data items was 0.99 and each data element with a value equal to or higher than 0.99 was considered a minimum data item.
Identification of Available HSCT Minimum Dataset
The literature review phase's output included the HSCT national and international registries. Most national registries collaborate with the related global registries in the world. For example, most European countries submit their HSCT data nationally to the EBMT registry. Therefore, based on the literature review phase, the regional and international registries are summarized in Table 5.
| No | Covered countries | Established year | Scope | Registry name | Abbreviation | More Info | Records number |
|---|---|---|---|---|---|---|---|
| 1 | Europe and International | 1974 | International | European group for Blood and Marrow Transplantation | EBMT | https://www.ebmt.org/ | 41 |
| 2 | United states and International | 1972 | International | The Center for International Blood and Marrow Transplant | CIBMTR | https://www.cibmtr.org/ | 57 |
| 3 | International | 2007 | International | Worldwide Network for Blood and Marrow Transplantation | WBMT | https://www.wbmt.org/ | 1 |
| 4 | Asia and Pacific | 1990 | Regional | The Asia Pacific Blood and Marrow Transplantation | APBMT | https://www.apbmt.org/ | - |
| 5 | Eastern Mediterranean | 2008 | Regional | The Eastern Mediterranean Blood and Marrow Transplantation | EMBMT | http://www.embmt.org/ | - |
| 6 | Latin American | - | Regional | Latin American Bone Marrow Transplantation Group | LABMT | https://www.wbmt.org/member-societies-of-wbmt/labmt/ | - |
| 7 | Australia and New Zealand | 1992 | National | Australasian Bone Marrow Transplant Recipient Registry | ABMTRR | www.abmtrr.org | 8 |
| 8 | Japan | 1978 | National | Japan Society for Hematopoietic Cell Transplantation registry | JSHCT | https://www.jshct.com/ | 12 |
| 9 | Britain | 1995 | National | British Bone Marrow Registry | BSBMT | https://bsbmtct.org/ | 7 |
| 10 | Canada | 1990 | National | Cell Therapy Transplant Canada (CTTC, formerly CBMTG) | CBMTG | https://www.cttcanada.org/page/BMTCentres | - |
| 11 | Italy | 1987 | National | Italian Blood and Marrow Transplantation Society | GITMO | https://www.gitmo.it/ | 5 |
| 12 | Germany | 1998 | National | German stem cell transplant registry | DRST | http://www.drst.de/drst/ | 3 |
| 13 | Poland | 2005 | National | - | Poltransplant | http://www.poltransplant.org.pl/ | |
| 14 | Malaysia | 1998 | National | National Transplant Registry | NTR | http://www.mst.org.my/ | |
| 15 | India | 2004 | National | Indian stem cell transplant registry | ISBMT | 1 | |
| 16 | South Korea | 1996 | National | The Korean Society of Blood and Marrow Transplantation | KSBMT | http://www.bmt.or.kr/eng/ | |
| 17 | Spain | 1990 | National | GELTAMO | https://www.geltamo.com/ | 3 | |
| 18 | Switzerland | 1997 | National | Swiss Transplant Working Group Blood and Marrow Transplantation Registry | STABMT | - | 2 |
| 19 | Taiwan | 1992 | National | Taiwan Bone Marrow Transplant Registry | TBMT | ||
| 20 | France | - | National | - | SFGM-TC | https://www.sfgm-tc.com/ | 4 |
| 21 | South Korea | 1996 | National | The Korean Society of Blood and Marrow Transplantation | KSBMT | http://www.bmt.or.kr/eng/ | - |
| 22 | Austria | - | National | Austrian Stem Cell Transplantation Registry (national registry in EBMT) | - | 1 | |
| 23 | South Africa | National | South African Bone Marrow Registry | SABMR | - | 3 | |
| 24 | China | - | National | Chinese Bone Marrow Transplantation Registry Group | CBMTRG | - | 5 |
| 25 | Czech | - | National | Czech National Registry | - | - | 1 |
| 26 | Taiwan | 1992 | National | Taiwan Bone Marrow Transplant Registry | TBMTR | 3 | |
| 27 | Brazil | - | National | Brazilian bone marrow registry | - | 1 |
Table 5: Identified Registries Based on Search.
Based on the finding presented in Table 5 and considering regional and international websites and their MDS, the experts' panel selected two known global registries, CIBMTR and EBMT, with a comprehensive minimum dataset and the highest number of countries collaborated with them. Based on the documents, 47 and 70 countries contribute data to the CIBMTR and EBMT, respectively. So, the essential data items of CIBMTR and EBMT, were considered in the current study.
The Final Version of MDS Development
The essential dataset for the HSCT registry was extracted and collected from the two selected international registries, the EBMT and the CIBMTR. This MDS has two categories: Administrative and clinical. The pre-HSCT information has administrative and clinical data. This questionnaire includes 146 accepted data items. In addition, seven questionnaires were created for the disease information, which has 698 accepted data items and the post-HSCT questionnaire consists of the follow-up information, which has 333 accepted data items which are presented in Table 6. The complete MDS Table is presented in Appendix 1. Table 7 shows the data item details of each section of MDS. Following Table 7 is a sample of the pre-transplantation of the pre-HSCT section.
| No | Total data items | Total data items | Accepted data items | Rejected data items | |
|---|---|---|---|---|---|
| 1 | Pre-HSCT | 225 | 147 | 78 | |
| 2 | Required data based on Disease | Leukemia | 358 | 233 | 125 |
| 3 | Lymphoma | 158 | 135 | 23 | |
| 4 | Myelodysplastic syndrome | 119 | 89 | 30 | |
| 5 | Myeloproliferative Neoplasms | 154 | 91 | 63 | |
| 6 | Myelodysplastic syndrome/Myeloproliferative Neoplasms | 43 | 35 | 8 | |
| 7 | Plasma cell disorders, including Multiple Myeloma | 108 | 80 | 28 | |
| 8 | Hemoglobinopathy | 45 | 35 | 10 | |
| 9 | Post-HSCT | 366 | 333 | 33 | |
| Total MDS items | 1576 | 1178 | 398 | ||
Table 6: MDS questionnaire.
| Pre-Transplantation | |||||
|---|---|---|---|---|---|
| No | Question | CIBMTR | EBMT | CVR | Accept/Reject |
| 30 | First transplant for this patient? | â?? | â?? | 1 | Accept |
| 31 | Number of prior HSCTs | â?? | 1 | Accept | |
| 32 | Type of prior HSCT | â?? | â?? | 1 | Accept |
| 33 | Date of prior HSCT | â?? | â?? | 1 | Accept |
| 34 | Was the prior HCT performed at a different institution? | â?? | â?? | 1 | Accept |
| 35 | Has patient or partner become pregnant after prior transplant? If yes go to next question | â?? | â?? | 0.6 | Reject |
| 36 | Did the pregnancy result in a live birth? | â?? | 0.6 | Reject | |
| 37 | Was the patient or partner pregnant at any time in this reporting period? | â?? | 0.6 | Reject | |
| 38 | Reason: Graft failure/insufficient hematopoietic recovery | â?? | 1 | Accept | |
| 39 | Reason: Persistent primary disease | â?? | 1 | Accept | |
| 40 | Reason: Recurrent primary disease | â?? | 1 | Accept | |
| 41 | Reason: Planned subsequent HCT, per protocol | â?? | 1 | Accept | |
| 42 | Reason: New malignancy (including PTLD and EBV lymphoma) | â?? | 1 | Accept | |
| 43 | Reason: Insufficient chimerism | â?? | 1 | Accept | |
| 44 | Reason: Other | â?? | 1 | Accept | |
| 45 | Date of Graft failure/rejection | â?? | 1 | Accept | |
| 46 | Date of relapse | â?? | 1 | Accept | |
| 47 | Date of secondary malignancy | â?? | 1 | Accept | |
| 48 | Specify other reason | â?? | 1 | Accept | |
| 49 | Has the recipient ever had a prior cellular therapy? | â?? | 0.6 | Reject | |
| 50 | Date of the prior cellular therapy | â?? | 0.6 | Reject | |
| 51 | Source for the prior cellular therapy | â?? | 0.6 | Reject | |
Table 7: Pre-transplantation part of the pre-HSCT section as a sample of MDS.
Among the international and regional registration systems, the CIBMTR registration system and the EBMT Registration System are well known HSCT registry systems founded in 2004 and 1974. Now, they register hematopoietic stem cell transplants from all over the world. Members of the European society of blood and bone marrow transplantation include Austria, Belgium, Denmark, Finland, France, Czech Republic, Germany, Greece, Ireland, Israel, Netherlands, Italy, Norway, Spain, Switzerland, Sweden, United Kingdom, Hungary, Turkey, Argentina, Singapore, Saudi Arabia, Slovenia, South Africa and Lebanon. However, some European countries, such as the United Kingdom and Germany, also have separate national registries, such as the British Bone Marrow Registry (BBMR) and German Stem Cell Transplantation Registry (DRST). Another regional-international center to collect and maintain HSCT data, CIBMTR, was created to improve transplant patients' survival, treatment and quality of life. The CIBMTR registry collects allogeneic transplantation outcome data mandatory and autologous transplantation outcome data from volunteers in the U.S. In addition, it also manages and maintains the allogeneic and autologous transplantation data from other countries worldwide volunteer. Up to now, in the CIBMTR registry, 575000 HSCT recipients' data are registered and more than 500 stem cell transplant centers around the world register transplant information in the U.S. international bone marrow transplant research center registration system. There are two active systems in the registration of bone marrow and stem cell transplantation among the regional registration systems. The Eastern Mediterranean bone marrow transplantation system was launched in the Eastern Mediterranean region in 2008 and now registered and maintains records of HSCT data from Algeria, Egypt, Iran, Jordan, Lebanon, Morocco, Oman, Pakistan, Saudi Arabia, Syria, Tunisia and Qatar centers. The Asia-Pacific bone marrow transplant registration system, in collaboration with Asia- Pacific, was established in 1990 and now records transplant information from the transplant centers of Australia/New Zealand, Bangladesh, Cambodia, China, Hong Kong, India, Indonesia, Iran, Japan, Myanmar, Pakistan, Philippines, Singapore, Sri Lanka, Taiwan, Thailand, Vietnam and Mongolia. These international and regional systems' Minimum Data Set (MDS) was examined. MDS of South Asian and Eastern Mediterranean transplant registration systems almost exists in the EBMT minimum data set. The most comprehensive datasets belong to the EBMT and CIBMTR Systems. The data items of these two international registry systems were compared and presented in Appendix 1. Each system's number and data items are different based on its purpose and mission. However, given that both the CIBMTR and EBMT systems have been developed to advance stem cell therapies and conduct research, in general, these two systems all have approximately the same coverage. In both systems, pre-transplant information, disease information and posttransplant information are recorded on day zero, 100 days after transplant, six months after transplant and annually. The two registers differ in structure and data elements in several respects: In the EBMT registry, in addition to registering MDS and MPN disease information, it is also possible to record disease information related to the combination of MDS/MPN and EBMT-MDS has its questionnaire. In comparison, the particular questionnaire on MDS/MPN disease combination is not available in the CIBMTR dataset. The data elements of the designed system also include items related to the combination of MDS/MPN disease. In the EBMT registry, the bone marrow failure syndrome information questionnaire includes acquired and congenital diseases of the bone marrow failure syndrome. Still, in the CIBMTR registry, the information questionnaire comprises the form of hereditary bone marrow failure syndrome and aplastic anemia. Because hematopoietic stem cell transplants for patients with this syndrome are not performed at the Imam Khomeini hospital complex transplant center, items related to this disease were removed. One of the differences in recording pre-transplant information in the two registries with the developed registry in this research is the number of recording chromosomal and molecular analysis information. In the CIBMTR registry, information related to chromosomal analysis and molecular analysis of patients is often recorded three times:
• At diagnosis.
• At the time between diagnosis and the start of regimen preparation or the last evaluation.
• At the time of the last evaluation.
Whereas in the EBMT registry, this information was recorded at diagnosis. In Imam Khomeini hospital complex transplant center, the mentioned analyzes, if possible, are performed only before the transplantation and registered only once. In the CIBMTR registry in chromosomal analysis, the results of the FISH and Karyotype methods are recorded separately by the technique. In contrast, in the EBMT registry, the results of either the FISH or Karyotype methods can be recorded without specifying the exact type of method. In the designed system, the information containing the separation of this method has been removed by obtaining a CVR of less than 0.99. In the CIBMTR system, out of 777 given items, 751 items were collected and registered. In the EBMT system, 657 required data items have been collected and combined with other elements. In total, a checklist and a questionnaire containing 1576 data elements have been obtained, 399 of which, due to the existing limitations and comments and reviews performed by the expert team, gained CVR with less than 0.99 and were removed and 1177 items were approved for the design of the registration system.
An essential data set for the HSCT registration system needed in the Imam Khomeini hospital complex transplant center in Iran was created and validated for system development. The MDS has nine sections: Pre-HSCT, leukemia, lymphoma, myelodysplastic syndrome, myeloproliferative Neoplasms, combined myelodysplastic syndrome and myeloproliferative neoplasms, Plasma cell disorders including Multiple myeloma, hemoglobinopathy and post HSCT. Data items were extracted from the CIBMTR and the EBMT essential dataset and investigated based on the experts' panel. This is the first step of registration system design and development. The registration system facilitates research to improve transplantation procedures, increase efficiency and survival rates and reduce post-transplant complications and mortality rates.
Ethical approval and consent to participate: This study was approved by the local Ethics Committee of TUMS with the code: IR.TUMS.SPH.REC.1399.043.
All authors have consent for publishing the manuscript in the BMC health and quality of life outcomes journal.
All available data for this study are included in the manuscript.
The authors have no competing interests as defined by BMC or other interests that might be perceived to influence the results and/or discussion reported in this paper.
This research received no specific grant from any funding agency in the public, commercial or not for profit sectors.
M Taheriyan and SR Niakan Kalhori and M Dabiri significantly contributed to designing the research study and searching literature. M Taheriyan and SR Niakan Kalhori and M Dabiri screened results, selected relevant papers and extracted data independently. M Taheriyan wrote the first draft of the manuscript and provided the figure and all tables. SR Niakan Kalhori critically edited and improved. SR Niakan Kalhori, N Mohammadzadeh and SR SafaeiNodehi reviewed the manuscript and took part in scientific advice and guidance.
Citation: Taheriyan M, Dabiri MZ, Nodehi SRS, Kalhori SRN, Mohammadzadeh N (2023) Minimum Dataset Development for Hematopoietic Stem Cell Transplantation (HSCT) Recipient Registry in Iran. Adv Appl Sci Res. 14:107
Copyright: © 2023 Taheriyan M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.