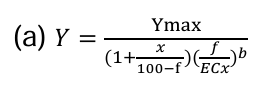Research Article - (2025) Volume 9, Issue 1
Microplastics Affect Microbial-Mediated Detoxification of Mercury in Aquatic Systems
Neusa Figueiredo1*,
Laura Silva1,
Vasco Branco2,
Cristina Carvalho2 and
Marta Martins1
1Department of Sciences and Environmental Engineering, NOVA School of Science and Technology, Caparica, Portugal
2Department of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
*Correspondence:
Neusa Figueiredo, Department of Sciences and Environmental Engineering, NOVA School of Science and Technology, Caparica,
Portugal,
Email:
Received: 12-Feb-2024, Manuscript No. IPJAPT-24-19045;
Editor assigned: 15-Feb-2024, Pre QC No. IPJAPT-24-19045 (PQ);
Reviewed: 29-Feb-2024, QC No. IPJAPT-24-19045;
Revised: 12-Mar-2025, Manuscript No. IPJAPT-24-19045 (R);
Published:
19-Mar-2025, DOI: 10.36648/2581-804X.9.1.36
Abstract
The coexistence in aquatic systems of chemical and physical contaminants, such as Mercury (Hg) and Micro-plastics (MPs, Ø <5 mm), is an intricate problem. Mercury is well known for its toxicological effects and MPs, due to their polymeric properties, can interact with chemicals, such as Hg, by absorbing them in their surface. Beside this, MPs can act as a surface of attachment for microorganisms. Knowing that, microorganisms are the key player in Hg natural detoxification processes in aquatic systems, the following questions arise: Does the presence of MPs affect microbial-mediated Hg-detoxification? To answer this, Hg-resistant microorganisms were isolated from Hg-contaminated area of the Tagus estuary and the most Hg-resistant strains were selected to assess the effect of polystyrene MPs (PS-MPs) on the Hg-detoxification process. After up to 5 days of incubation with Hg and Hg plus PS-MPs, culture medium and PS-MPs were collected to assess the: 1) Hg-removal; 2) Acute toxicity of the supernatant; 3) pH variations of the supernatant and 3) Bacteria association with PS-MPs. The isolates selected for this study (strains PWLO and PWBL) were identified through the amplification of 16S rRNA as Pseudomonas sp. The results demonstrated that: (i) Both strains are resistant to Hg and can remove Hg2+ from the liquid medium and (ii) The presence of PSMPs affects Hg-removal mediated by the strain PWLO and consequently the detoxification processes. Thus, this set of data highlight the impact that PS-MPs may have on Hg natural-occurring detoxification process mediated by Hg-resistant bacteria in aquatic ecosystems.
Keywords
Hg2+; Polystyrene microplastics; Pseudomonas; Detoxification
Introduction
Plastic debris of various sizes, including macro-plastic (<1 m), meso-plastic (2.5 cm-5 mm) and micro-plastic (MPs, Ø <5 mm), are ubiquitous in aquatic systems, including freshwater, estuaries, coastal and open sea, Antarctica and are known to interact with aquatic organisms from all trophic levels, resulting in a range of toxicological effects. Especially, in recent years, MPs are the most common plastic debris reported in aquatic systems and studies have been demonstrated toxicological effects on a wide range of aquatic biota, from mussels, freshwater bivalve to fishes [1].
MPs can be classified as primary MPs (Ø from 0.1 to 5.0 mm), if deliberately manufactured for commercial use (e.g., plastic pellets, micro-beads from personal care products, paint flakes) or secondary MPs, if resulting from the fragmentation of larger plastic items via UV solar radiation, chemical degradation and biodegradation. Compared with large plastic debris, MPs are of special concern, because of their bioaccumulation potential, which increases with the decreasing on size.
Once inside aquatic systems, MPs are known to be taken up, ingested and accumulated throughout the aquatic food chain at all levels. MPs alone have been shown to cause oxidative stress, neurotoxicity, genotoxicity and inflammation. In addition, due to their large surface-to-volume ratio and lipophilicity, MPs can interact with other environmental pollutants, including metals, pharmaceuticals, polycyclic aromatic hydrocarbons, etc. in a size-dependent way.
Besides chemical adsorption, there are also microorganisms developing biofilms on the surface of MPs polymers, the socalled “plastisphere”. These include huma and animal pathogens (e.g., genus Vibrio) and hydrocarbon-degrading bacteria, which may potentially influence plastic debris fragmentation and degradation. However, little is known about the ecological functions of this community [2]. In the case of marine plastisphere, it is already known that these microorganisms play a key role in the biogeochemical cycles in the oceans. Moreover, recently Seeley, et al., reported that the presence of MPs alters sediment microbial community composition and nitrogen cycling processes. By comparing sediments without MPs and sediment with MPs, the authors found that some MPs promote nitrification and denitrification, while others (e.g., polyvinyl chloride) inhibit both processes. Still, the effect of MPs on microorganisms/microbial communities living in aquatic systems and their functions are poorly understood and need to be further investigated.
Industrialized and urbanized coastal areas, including estuarine ecosystems, are known as the hotspots of anthropogenicoriginated contamination with several ubiquitous pollutants, including MPs and Mercury (Hg). Hg is among the 10 chemicals of major public health concern (WHO), exhibiting toxicity even at low concentrations. In the aquatic systems, inorganic Hg is converted into the highly neurotoxic Methyl Mercury (MeHg), which is biomagnified along the food chain. In these systems, the bio-methylation of mercuric mercury, i.e. carried out by microorganisms, is the major pathway responsible for high concentrations of MeHg. The coexistence of Hg and MPs in the aquatic system is a complex problem, as toxic effects, including increased toxicity resulting from the simultaneous exposure to MPs and Hg, have been reported.
In our previous studies, it was shown that microorganisms, namely bacteria, are involved in the biogeochemical cycle of Hg in Tagus estuary, an estuary showing high to moderate levels of these metals, including the MeHg. Aerobic bacteria community and isolates were shown to be involved mainly in processes of Hg-detoxification, including Hg-reduction and MeHg-demethylation [3]. Thus, knowing that Hg can be adsorbed to MPs surface and may interact with aquatic microorganisms from the plastisphere, we aim to clarify the repercussion of these interactions on the biogeochemical cycle of Hg to answer the question: Do microplastics affect microbialmediated processes taking place in Hg-biogeochemical cycle? For this purpose, Polystyrene (PS) polymer particles (PS-MPs) were used as an MPs model for this study and Hg-resistant bacteria were isolated from water samples collected from a Hgcontaminated area of Tagus Estuary (Barreiro-industrial area).
Material and Methods
Tested Substances and Other Chemicals
To obtain the PS-MPs, PS commercial plastic materials were powdered, producing particles with a size ≤ 1 mm. PS (C8H8) is a synthetic aromatic hydrocarbon polymer, which is hard, rigid, solid at room temperature and transparent. This polymer is an appropriate material for the food container and for the packaging of industrial products. Mercury chloride (HgCl2) (Riedel-de Haen) (99.9%) was used in all Hg-containing assays. The bacterial culture was maintained in Mueller- Hinton (MH) (Biokar diagnostics) medium, a testing medium recognized as standard by NCCLS [4].
Sediments and Water Samples Collection
According to previous studies, a contaminated area of Tagus estuary was selected for this study-Barreiro (Lat: 38.664500, Long: -9.078600) (Figure 1). This specific area of Tagusestuary is a well-known highly impacted area with an intensive industrial activity (e.g., metal-mechanical and textile activities) since the 1960 decade. Although Hg has been phased out from industrial activities, a high level of Hg contamination still exists.
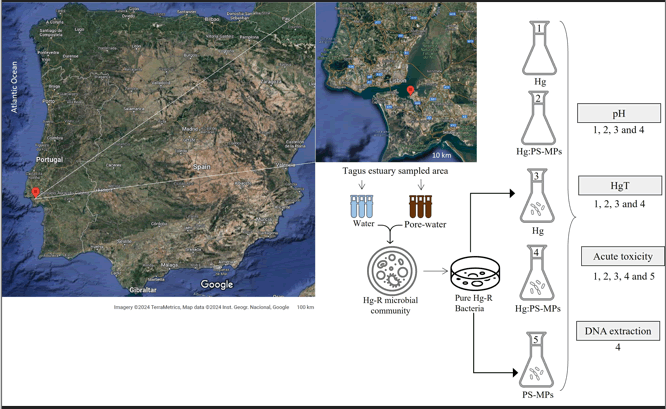
Figure 1: Tagus estuary sampled area (Lat: 38.664500, Long: -9.078600) and schematic representation of bioassay set up to evaluate the effect of Polystyrene Microplastics (PS-MPs) on microbial-mediated Hg-reduction. Two highly Hg-33 resistant strains isolated from Tagus estuary were used (PWBL and PWLO) in the assays 3, 4 and 5. Assay 1 was performed as Hg control and assay 2 was performed to investigate the interaction between Hg and PS-MPs. The endpoint evaluated was the pH, HgT, toxicity test and PS-MPs-associated DNA.
Sediment samples (3 cm-deep) were collected to sterile centrifuge tubes in triplicate at the intertidal zone, during the spring season at low tide. Water samples were collected in triplicate near the shoreline directly into a sterile centrifuge tube (50 ml). The tubes containing both water and sediment samples were sealed and stored in a refrigerated chamber and transported to the laboratory. The sediments were centrifuged at 16,582 × g for 2 minutes and the pore water was collected and stored for microbial community isolation.
Bacteria Isolation and Mercury Susceptibility Testing
Bacteria were isolated from the water and pore water samples. Eighteen inoculums were prepared by diluting 1 ml of each triplicated water sample into 9 ml of distilled sterile water. To isolate Hg-resistant/tolerant bacteria, 500 μl of each inoculum were plated on MH agar media with and without Hg2+ selective pressure (5.0 μM) and incubated at room temperature [5]. After three days of incubation, the different bacterial colonies observed were isolated into a new plate with the same Hg selective pressure. A total of 12 pure colonies were isolated and stored in a refrigerated environment.
The Hg-resistance levels of each isolate were inferred from the Minimal Inhibitory Concentration (MIC) determination, using a modified micro-dilution broth method described by clinical laboratory standards institute. Briefly, bacteria cultures in MH broth at a concentration of approximately 108 Colony-Forming Units (CFU)/ml were diluted in MH broth to obtain 106 CFU/ml. In a sterile 96-well micro-plate, 100 μl of this bacterial suspension were placed and afterward, 100 μl of aqueous HgCl2 solution were added into the wells of the first column. Then, sequential dilutions (1:2) were performed in the following column until eleventh column to obtain concentrations ranging from 0.10 to 500 μM Hg2+. Bacterial suspension in the absence of Hg (twelfth column) was used as a negative control [6]. Duplicate samples were performed for each concentration tested. After incubation at room temperature in the dark, for 24 h and under aerobic conditions, the presence or absence of bacterial growth was observed. The MIC was defined as the minimum concentration of test compound that inhibited visible growth. All data points represent the mean ± Standard Deviation (STD) of 3 independent determinations (each one performed with duplicates).
Bioassay Setup
Among the Hg-resistant isolates, two highly resistant strains (PWBL and PWLO) were selected to study their Hg2+ removal capacity and the effect of PS-MPs on this process. To investigate the correlation between microbial-mediated Hgremoval and the effects of PS-MPs on this process, a setup of the assay with five conditions was designed: (1) Hg; (2) PS plus Hg (Hg: PS-MPs); (3) The selected strains plus Hg (PWBL: Hg and PWLO: Hg); (4) The selected strains in Hg and PS-MPs spiked medium (PWBL: Hg: PS-MPs and PWLO: Hg; PS-MPs) and (5) the selected strains in PS spiked medium (PWBL:PSMPs and PWLO:PS-MPs) (Figure 1).
The incubation medium was 1:1 proportion of sterile river water and 50% of MH broth. This medium was spiked with 2 μM of inorganic Hg ([Hg2+]>EC50 (0.64 ± 0.02 μM)) (a preliminary result of this study). The PS-MPs were adjusted to 40 mg/L, as the reference concentration, taken from the highest MPs concentration used in a bioassay by Prata, et al. The assay was carried out in duplicate, at room temperature [7]. Three independent assays were performed. After incubation for up to 5 days, 5 ml of culture medium were centrifuged at 16,582 × g for 1 min to separate the supernatant from the cell pellet. The supernatant was used for determination of Total Hg (HgT), pH measurement and Microtox analyses. The pellet was used for HgT measurement. PS-MPs were also collected from each treatment through suspension filtration using a 200 μm filter for DNA extraction.
Determination of HgT in the Collected Samples
The concentration of HgT was determined in both water and pore water samples collected from the sampled area (Barreiro) and in the supernatant and pellet samples collected from the bioassays. The measurement was performed via atomic absorption spectrometry, using a silicon Ultraviolet (UV) diode detector LECO AMA-254 as previously described. Precision, expressed as the relative standard deviation of 3 replicate samples (2 measurements for each one), was 2.4% [8]. Spiked Hg solution (5.0 μM) was used to ensure the accuracy of the procedure and the obtained value was 4.90 ± 1.16 μM (not statistically different). Media plus Hg was used as the negative control of the bioassay. Three independent assays were performed and data are expressed as Hg variation relative to the negative control.
pH Analysis
The pH of each supernatant samples collected from the bioassay was measured with a benchtop pH/mV/â?? meter, phenomenal® pH 1100L, calibrated with technical buffers pH 4.01 and pH 7.00 (VWR)
Microtox Analyses
The evaluation of the toxicity associated to the 5-days old supernatant samples was carried out according to a standard ISO toxicity laboratory method, 11348-3: 2007, the Microtox acute toxicity test. This test was used to evaluate the bioluminescence inhibition of Allivibrio fischeri after exposure to the supernatant samples [9]. Microtox test is a rapid, simple, cost-effective and generally as sensitive as fish and invertebrate toxicity assays approach that uses freeze-dried bacteria and allows determining the ECx (Effect Concentration) of a compound or environmental sample. The analyses were carried out using a Microtox Model 500 toxicity analyzer system (Modern Water) and the procedure was following the MicrotoxOmni software (version 4.3, 1995, Los Angeles, CA, USA). The resulting data was used to calculate EC20.
DNA Extraction and Bacteria Identification
Bacterial DNA associated with the PSMPs and from pure colonies isolated from pore water were extracted by using the DNeasy power water kit (Qiagen) according to manufacturer’s instructions. The DNA was quantified by a nano-drop spectrophotometer and expressed as ng/μL [10].
For the identification of isolates, the 16SrRNA gene was amplified using 16SV1-V3 primers (F28- GAGTTTGATCNTGGCTCAG/R519-GTNTTACNGCGGCKGCTG). Polymerase Chain Reaction (PCR) reactions were carried out in 25 μl volume containing 12.5 μl of PCR master mix (50 mM Tris-HCl, pH 9, 50 mM NaCl, 2.5 mM MgCl2, 200 μM of each dNTP and 0.2 U/μl of NZYTaq DNA polymerase), 8.5 μl of nuclease-free water, 0.1–1 μM of primers and 0.05–0.5 μg of template DNA. PCR mixtures were amplified by initial holding at 98°C for 30 s and then 20-35 cycles of denaturing at 98°C for 10 s, annealing at 58°C for 30s and extension at 72°C for 30s and a final extension at 72°C for 10 min. The sizes of amplicons were confirmed by gel electrophoresis. PCR products were purified using the NZYGelpure kit (NZYTech), following the protocol instructions. Sequencing was performed by STAB-Vida (Lisbon, Portugal), using the same primers used for amplification.
All sequences were subjected to BLAST search for comparison with published sequences. Multiple alignments with the known 16S rRNA gene sequence from GenBank were performed by the CLUSTAL W2 algorithm.
Statistical Analysis
All the data are presented as mean ± standard deviation (n=3 independent assays). Differences between experimental groups were determined by applying the Fisher Test and t-test for independent samples, considered significant at a p-value <0.05. ANOVA PostHoc-Dunnett test was as well applied (pvalue <0.05) [11].
Statistics were performed using the software SPSS (IBM SPSS Statistics 25). In order to calculate the ECx of A. fischeri bioluminescence inhibition, the datasets were fitted to a 3- parameter logistic dose-response model.
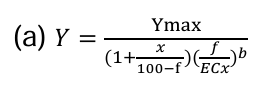
In this equation, Y is the A. fischeri bioluminescence, Ymax is the Y maximal, x is the percentage of the effect, f is the percentage of the supernatant sample, the ECx is the effect concentration for x and b is the slope. The calculations were performed using SPSS (IBM SPSS statistics 25).
Results
HgT in Water and Pore-water Samples of Tagus Estuary
The HgT concentrations in water and pore-water samples are presented in Table 1. Both samples showed similar levels of Hg, 0.42 μM and 0.65 μM, respectively.
Hg-resistant Isolates
The number of colonies resulting from the inoculums of water and pore-water samples spread into the medium with and without Hg selective pressure (5.0 μM Hg2+) is shown in Supplementary Table 1. Medium with selective pressure showed a higher number of colonies and porewater samples exhibited more colonies in both conditions, compared with the water samples (Supplementary Table 1) however, there is no significant difference between them (p <0.05). In terms of diversity (different colonies), eleven different colonies were found on water samples and seven different colonies were found on pore-water samples (Supplementary Table 1). The presence of Hg decreased the diversity on pore-water samples (Shannon index for media without Hg>Shannon index for media with Hg) but not for water (Shannon index for media without Hg ≈ Shannon index for media with Hg) (Table 1) [12].
| Samples |
HgT (µM) |
Shannon H index |
MIC values (µM) |
| w/o Hg |
w/ 5 µM Hg |
| Water |
0.42 ± 0.02a |
1.94 |
1.95 |
4.00-31.4b |
| Pore-water |
0.65 ± 0.01a |
1.26 |
0.65 |
85.8-125b |
| Note: a and b refers to the significant differences between the samples (p-value<0.05). |
Table 1: Total Hg concentration (HgT) in water samples of Tagus Estuary (Barreiro) and diversity index of Hg-resistant bacteria colonies isolated from these samples. The minimal inhibitory concentration (MIC) reflects the Hg-resistance level of each isolate.
A total of twelve different colonies were found in media containing 5.0 μM Hg2+ (Supplementary Table 1), from both water and pore-water samples. From these, a total of four different colonies (two from water and 2 from pore-water samples) were selected for the subsequent experiment, according to their dominance in the medium (Supplementary Table 1). The MIC values for these four selected bacterial strains ranged from 4.0 to 125 μM (Table 1). In general, the pore-water isolates showed higher MIC values, in comparison with the homologous water sample isolates, in particular the PWBL strain with a MIC value as high as 125 μM. Therefore, due to its greater resistance to Hg, the pore-water isolates (PWBL and PWLO) were selected to be used in the assays to assess their Hg-reduction potential and the influence of PSMPs in this process [13].
The selected microorganisms were identified through 16S rRNA gene amplification. All the isolates correspond to the genus Pseudomonas with 99-100% of identity (Table 2). The DNA extracted from PS-MPs after five days of incubation with the selected microorganisms was quantified and the trend was PWLO: PS-MPs (2.50 ng/μL) >PWBL:PS-MPs (0.70 ng/μL). The amplified 16S rRNA genes sequence obtained from this DNA had a 99-100% match with the genus Pseudomonas (Table 2).
| DNA extracted from |
Isolates |
Identification based on 16SrRNA |
% identity |
Accession no. |
| Liquid media |
PWBL |
Pseudomonas sp. |
99 |
MK478928 |
| PWLO |
Pseudomonas sp. |
100 |
AM778697 |
| Microplastics |
PWBL |
Pseudomonas sp. |
100 |
AM778697 |
| PWLO |
Pseudomonas sp. |
99 |
MK729053 |
Table 2: Identification of bacteria isolates based on 16SrRNA, there percentage 701 identity and accession numbers.
Bacterial-Mediated Hg-Removal
The HgT concentrations were determined in the supernatant and cell pellet fractions after 24 h of incubation, to evaluate bacterial-mediated Hg-removal [14]. Figure 2 portrays the mass balance of HgT (Figure 2) and the fold change relatively to the control (HgT in the supernatant), after 24 h of incubation of both selected strains with 5.0 μM of Hg2+. On the first day of incubation (24 h of incubation), the medium containing the isolates and Hg (PWBL: Hg and PWLO: Hg) showed a clear decrease in HgT concentration (Figure 2A). Compared with the control (medium containing solely Hg), the HgT in PWBL: Hg and PWLO: Hg supernatant are statistically significant (p-value <0.05). Considering the mass balance, it can be observed that 57 and 38% of Hg were missing after the first day of incubation with these two strains (PWBL and PWLO strains, respectively). No significant differences were found between the two strains (p-value <0.05).
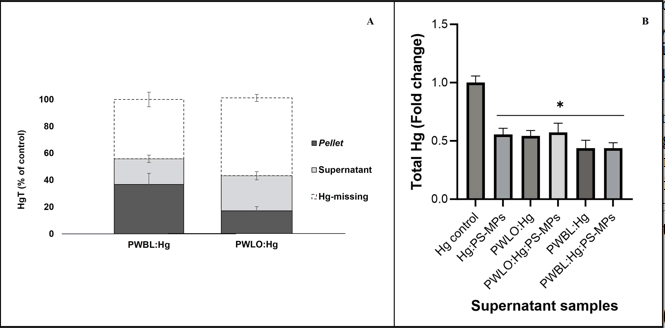
Figure 2: Mass balance of Total Hg (HgT) (A) and the variation of Total Hg (HgT) measured on the supernatant (B), after 24 h of incubation at room temperature. (A) HgT was measured on cell pellet and supernatant samples collected after one day of incubation of the strains PWBL and PWBL with 5.0 μM of Hg2+. The results are presented as a percentage of Hg control (% of control). (B) Fold change from the Hg control (mediumplus 5 μM Hg2+) of total Hg (HgT) measured on the supernatant samples of 6 conditions: Hg, Hg plus PS-MPs (Hg:PS-MPs), PWLO strain plus Hg and plus Hg and PS-MPs (PWLO:Hg and PWLO:Hg:PS-MPs) and PWBL strain plus Hg and plus Hg and PS-MPs (PWBL:Hg and PWBL:Hg:PS-MPs). The culture medium (1:1 river water and MH broth) was spiked with 5 μM Hg2+ and 40 mg/l of PS-MPs Ø <1 mm. The data are expressed as the mean ± standard deviation of three independent assays. (*) indicate significant differences in comparison with Hg control (p-value <0.05).
Effects of PS-MPs on Hg Removal
The HgT of the supernatant was determined after 24 h and five days of incubation for conditions Hg:PS-MPs, PWBL:Hg:PS-MPs and PWLO:Hg:PS-MPs. Figure 2 shows the obtained results for 24 h. All three conditions are statistically different from the control (p-value<0.05), exhibiting Hg-removal from the culture medium. The presence of PS-MPs decreased the Hg removal mediated by the strain PWLO, being that the remaining HgT concentration in the supernatant of PWLO:Hg:PS-MPs condition was slightly higher than the PWLO:Hg, however this difference are not statistically significant (p-value<0.05) [15].
After five days of incubation, the trend of HgT concentrations was: PWLO:Hg:PS- MPs>Hg:PS-MPs>PWLO:Hg>PWBL:H>PWBL:Hg:PSMPs (Figure 3). The PWBL:Hg:PS-MPs supernatant exhibited the lowest HgT concentration (0.23 ± 0.01 μM), with a significant difference from the Hg:PS-MPs (p-value<0.05), while PWLO:Hg:PSMPs supernatant exhibited the highest HgT concentration (0.57 ± 0.02 μM), with a significant difference from Hg:PS-MPs and PWLO:Hg (p-value<0.05) (Figure 3). Significant differences were found between the two strains (p-value<0.05) (Figure 3). Lower than for the PWLO:Hg samples, the dose-response curves are not statistically different (p-value<0.05).
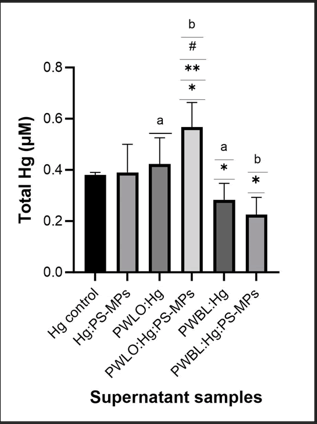
Figure 3: Variation of Total Hg (HgT) measured on the supernatant samples of Hg:PS-MPs, PWLO plus Hg and plus Hg and PS-MPs (PWLO:Hg and PWLO:Hg:PS-MPs) and PWBL strain plus Hg and plus Hg and PS-MPs (PWBL:Hg and PWBL:Hg:PS-MPs), after five days of incubation at room temperature. The culture medium (1:1 river water and MH broth) was spiked with 5 μM Hg2+ and 40 mg/l of PS-MPs Ø <1 mm. The values are expressed as fold change from the Hg control (medium-plus 5 μM Hg2+). (*), (**) and (#) indicates significant differences in comparison with Hg control, Hg:PSMPs and with the homologous supernatant of the strain plus Hg, respectively and (a) and (b) indicates differences between the two strains (p-value<0.05).
PH Variation on the Supernatant
The pH values of the supernatant after five days of incubation are shown in Figure 4. A change in the pH values would indicate that the chemical additives present in PS-MPs would be dissociating from it to the suspension.
The presence of PS-MPs on the medium led to a slight drop on the pH value (0.039 units) in comparison with the Hg control, however, without significant differences (p-value<0.05) [16]. Regarding media containing the bacteria strains, in both conditions with Hg and with Hg:PS-MPs, the pH values increased in comparison with the Hg control with significant differences (p-value<0.05) (Figure 4). No significant differences were found between the supernatants containing the isolates strains with and without PS-MPs.
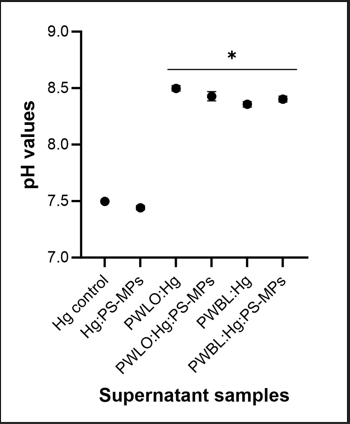
Figure 4: The variation of pH values of the supernatant containing Hg control, Hg:PS-MPs, Hg:PWBL, Hg:PWLO, Hg:PWBL:PS-MPs and HgPWLO:PS-MPs, after three and five days of incubation at room temperature. The data are expressed as the mean ± standard deviation of three independent assays. (*) indicates significant differences in comparison with Hg control (medium-plus 5 μM Hg2+) (pvalue <0.05).
Discussion
In the present study, the effect of PS-MPs on the Hg-removal potential of two Hg- resistant Pseudomonas isolated from an Hg-contaminated estuarine ecosystem was evaluated.
Highly Hg-resistant bacteria were isolated from water and pore-water samples of the Tagus estuary (Barreiro). This estuary and particularly this area on the south line, is known for its moderate-to-high levels of Hg contamination as a result of intensive past industrial activity. In fact, the results reported here for HgT on the two-sampled matrices (0.42 μM on water and 0.65 μM on pore-water) are higher than those reported by Canario et al. and Figueiredo et al., for porewater of the north channel (range 1.8–79 pM) and saltmarsh (mean 12 pM) areas of this estuary. This corroborates the higher Hg contamination found in this area [17].
The two selected Hg-resistant bacteria strains (PWLO and PWBL) are two Pseudomonas sp. exhibiting Hg-removal potential. Among them, the PWBL strain displayed a higher Hg removal capacity from the culture medium. However, the mass balance showed a higher Hg loss for the PWLO (57.4 ± 2.57 %), after 24 h of incubation, compared with the 44 ± 5.44 % for PWBL [18]. The lower Hg concentration in the medium inoculated with PWBL may be explained by the higher Hg uptake by cell fraction. The Hg-removal potential is likely the result of the higher Hg-resistance displayed by this strain. Previous studies conducted by Figueiredo, et al., reported the existence of highly Hg-resistant bacteria in Tagus Estuary, particularly in the sediment of the sampled area (Hg2+ MIC values ranging from 0.80 to 698 μM. These Hg-resistant bacteria were mostly Bacillus sp. and were found to perform Hg-reduction with subsequent volatilization of Hg0. It is plausible that a similar mechanism exists in these Pseudomonas sp. strains, thus explaining the consistent decrease of HgT observed in the supernatant.
On the other hand, when PS-MPs were added to the culture medium, the concentration of HgT in the supernatant was lower than the Hg-control, after 24h of incubation, but higher after 5 days of incubation. This may be related to the PS-MP absorption affinity to Hg, which may create a dynamic of absorption followed by desorption in Hg:PS-MPs. This result points that PS-MPs can interfere with the Hg-removal by decreasing the bioavailability of Hg to be reduced by bacteria in the culture medium. For instance, HgT in medium containing PWLO was higher when PS-MPs were added.
Other hypotheses for the interference of PS-MPs on the microbial-mediated Hg-removal may rely on the fact that microorganisms can attach to the PS-MPs surface. This is most likely in the case of strain PWLO. Indeed, the DNA quantity extracted from PS-MPs incubated with PWLO was almost four times higher than the concentration of PWBL:PS-MPs-associated DNA, indicating a higher ability of PWLO to be attached to the PS-MPs surface. The hydrophobic characteristic of PS-MPs promotes forces of attraction between microorganisms and this polymer surface, which may enable microorganisms to attach themselves to the polymer’s surface and to move from water to this subtract.
This is a significant result since it shows that the association between MPs and Hg-resistant bacteria compromises the detoxification process of a medium containing Hg, by reducing the available number of microorganisms to perform this process.
The interference of PS-MPs with Hg-removal may also affect the resulting toxicity. For instance, after five days of exposure, the toxicity of supernatant samples collected from the PWLO:Hg:PS-MPs treatment was higher than the PWLO:Hg supernatant. Despite there being no significant differences on a laboratory scale, one cannot be sure in the environmental scale and in long- term toxicity. In the opposite, the presence of PS-MPs did not interfere with the
Hg removal capacities of the PWBL strain. This strain showed a higher and faster Hg-removal capacity by accumulating Hg in cell pellet. This observation reveals that the effect of PS-MPs on bacterial-mediated Hg-removal will depend on the mechanisms involved in this process (Table 3) [19].
| Supernatant samples |
EC20 (% of supernatant) |
| Hg control |
2.5 (0< -8.4) |
| Hg:PS-MPs |
11.9 (1.0-22.8) |
| PWBL:Hg |
(n.d)1 |
| PWLO:Hg |
1.0 (0.1-1.3) |
| PWBL:Hg:PS-MPs |
(n.d)1 |
| PWLO:Hg:PS-MPs |
0.9 (0< -3.0) |
Table 3: Effect concentration EC20 (with 95% confidence intervals) of the 5-days older supernatant samples collected from the bioassay. The EC20 is the effect concentrations causing 20% of inhibition of A. fischeri bioluminescence, after 25 minutes of exposure of to the supernatant samples. The effect concentrations were obtained by the calculations using several dilutions of the supernatant (expressed as the percentage).
Concerning the pH variation, no significant variation was observed up to five days of incubation, when comparing the supernatant of medium with PS-MPs and without PS-MPs (Figure 4). This may indicate that the chemical additives present in PS-MPs were not dissociated from it to the suspension. The release of compounds with basic or acid properties would translate into an increase or decrease of the pH value, respectively. However, for a more conclusive result, the pH variation must be assessed for a longer exposure time. For instance, Auta, et al., reported a continuous increase in pH after 40 days of incubation of polypropylene with Bacillus sp. strain 27 and Rhodococcus sp. strain 36. The same was reported by Habib, et al., for the same period of incubation of polypropylene with Pseudomonas sp. ADL15 and Rhodococcus sp. ADL36. The authors suggested that this increase in pH towards a more alkaline state is related to the synthesis of bacterial exo-enzymes involved in the polymer deterioration [20].
Conclusion
This study is the first evidence of PS-MPs interference in Hgremoval mediated by aquatic microorganisms. This evidence was shown by using Pseudomonas sp. strains isolated from a Hg-contaminated area of Tagus estuary.
The analysis of water and pore-water samples collected from this historic industrial area proved that such contamination persists in the present time and that Hg contamination is still a problem in this estuary. Hg-resistant microorganisms were found to exist both in the water and pore-water samples of this area. The two Hg-resistant Pseudomonas sp. strains isolated from pore-water samples showed potential to remove Hg2+ from liquid medium up to 50%, which reinforces the detoxification potential of Tagus estuary’s microorganisms. However, the presence of PS-MPs may interfere with this process. Thus, it is important to know if this interference may increase the persistence of Hg in the aquatic systems and affect the methylation process. In this way, this study emphasizes the importance of future studies to assess the quantity and species of Hg adsorbed to MPs and the kinetics of adsorption and desorption of Hg and MeHg with MPs. In the context of environmental risk assessment, the impact of MPs on Hg bio-transformations, i.e., in the methylation, demethylation and reduction processes, taking part in an Hg-contaminated aquatic system, are needed.
Author Contributions
Neusa Figueiredo: Conceptualization, methodology, validation, investigation, supervision, writing–original draft, funding acquisition; Laura Silva: Formal analysis, investigation; Cristina Carvalho: Writing–review and editing; Vasco Branco: Resources, writing–review and editing; Marta Martins: Conceptualization, methodology, validation, supervision, resources, writing–review and editing, funding acquisition.
All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Portuguese foundation for science and technology, FCT, I.P. (http://www.fct.pt/) through marine and environmental sciences Centre-MARE (UIDB/ 04292/2020 and UIDP/04292/2020), the Project PLASTISPHERE (PT-IL/0001/2019), project PlasticHg (2022.04580.PTDC; DOI 10.54499/2022.04580.PTDC) and by iMed. ULisboa’s Strategic Project (UIDB/04138/2020; UIDP/ 04138/ 2020). L. Silva is grateful to MARE for her research scholarship “Verão com Ciência 2021” by FCT I.P.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors acknowledge Isabella Bramatti for her support in the analysis.
References
- Alsharaeh EH, Othman AA, Aldosari MA (2014) Microwave irradiation effect on the dispersion and thermal stability of RGO nanosheets within a polystyrene matrix. Materials. 7(7):5212-5224.
[Crossref] [Google Scholar] [PubMed]
- Antunes J, Frias J, Sobral P (2018) Microplastics on the Portuguese coast. Mar Pollut Bull. 131:294-302.
[Crossref] [Google Scholar] [PubMed]
- Auta HS, Emenike CU, Jayanthi B, Fauziah SH (2018) Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Marine Pollut Bull. 127:15-21.
[Google Scholar]
- Barboza LG, Vieira LR, Branco V, Figueiredo N, Carvalho F, et al. (2018) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol. 195:49-57.
[Crossref] [Google Scholar] [PubMed]
- Branco V, Caito S, Farina M, Teixeira da Rocha J, Aschner M, et al. (2017) Biomarkers of mercury toxicity: Past, present and future trends. J Toxicol Environ Health B Crit Rev. 20(3):119-154.
[Crossref] [Google Scholar] [PubMed]
- Canario J, Branco V, Vale C (2007) Seasonal variation of 520 monomethylmercury concentrations in surface sediments of the Tagus 521 Estuary (Portugal). Environ Pollut. 148(1):380–383.
- Canario J, Vale C, Caetano M (2005) Distribution of monomethylmercury and mercury in surface sediments of the Tagus Estuary (Portugal). Mar Pollut Bull. 50(10):1142-1145.
[Crossref] [Google Scholar] [PubMed]
- Canario J, Vale C, Caetano M, Madureira MJ (2003) Mercury in contaminated sediments and pore waters enriched in sulphate (Tagus Estuary, Portugal). Environ Pollut. 126(3):425-433.
[Crossref] [Google Scholar] [PubMed]
- Capolupo M, Sorensen L, Jayasena KD, Booth AM, Fabbri E (2020) Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 169:115270.
[Crossref] [Google Scholar] [PubMed]
- Castro-Ferreira MP, Roelofs D, van Gestel CA, Verweij RA, Soares AM, et al. (2012) Enchytraeus crypticus as model species in soil ecotoxicology. Chemosphere. 87(11):1222-1227.
[Crossref] [Google Scholar] [PubMed]
- Carvalho CM, Chew EH, Hashemy SI, Lu J, Holmgren A (2008) Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J Biol Chem. 283(18):11913-11923.
[Crossref] [Google Scholar] [PubMed]
- Cesario R, Mota AM, Caetano M, Nogueira M, Canario J (2018) Mercury and methylmercury transport and fate in the water column of Tagus estuary (Portugal). Mar Pollut Bull. 127:235-250.
[Crossref] [Google Scholar] [PubMed]
- Chagnon C, Thiel M, Antunes J, Ferreira JL, Sobral P, et al. (2018) Plastic ingestion and trophic transfer between Easter Island flying fish (Cheilopogon rapanouiensis) and yellowfin tuna (Thunnus albacares) from Rapa Nui (Easter Island). Environ Pollut. 243:127-133.
[Crossref] [Google Scholar] [PubMed]
- Clinical and laboratory standards institute (2012) methods for dilution 552 antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-Ninth Edition. CLSI document M07-A9; USA.
- Defontaine S, Sous D, Tesan J, Monperrus M, Lenoble V, et al. (2020) Microplastics in a salt-wedge estuary: Vertical structure and tidal dynamics. Mar Pollut Bull. 160:111688.
[Crossref] [Google Scholar] [PubMed]
- Figueiredo NL, Canario J, O Driscoll NJ, Duarte A, Carvalho C (2016) Aerobic mercury-resistant bacteria alter mercury speciation and retention in the Tagus Estuary (Portugal). Ecotoxicol Environ Saf. 124:60-67.
[Crossref] [Google Scholar] [PubMed]
- Figueiredo NL, Canario J, Serralheiro ML, Carvalho C (2017) Optimization of microbial detoxification for an aquatic mercury-contaminated environment. J Toxicol Environ Health A. 80(13-15):788-796.
[Crossref] [Google Scholar] [PubMed]
- Figueiredo NL, Canario J, Duarte A, Serralheiro ML, Carvalho C (2014) Isolation and characterization of mercury-resistant bacteria from sediments of Tagus Estuary (Portugal): Implications for environmental and human health risk assessment. J Toxicol Environ Health A. 77(1-3):155-168.
[Crossref] [Google Scholar] [PubMed]
- Figueiredo NL, Areias A, Mendes R, Canario J, Duarte A, et al. (2014) Mercury-resistant bacteria from salt marsh of Tagus Estuary: the influence of plants presence and mercury contamination levels. J Toxicol Environ Health. 77(14-16):959-971.
[Crossref] [Google Scholar] [PubMed]
- Figueiredo N, Serralheiro ML, Canario J, Duarte A, Hintelmann H, et al. (2018) Evidence of mercury methylation and demethylation by the estuarine microbial communities obtained in stable Hg isotope studies. Int J Environ Res Public Health. 15(10):2141.
[Crossref] [Google Scholar] [PubMed]
Citation: Figueiredo N, Silva L, Branco V, Carvalho C, Martins M (2025) Microplastics affect Microbial-Mediated Detoxification of
Mercury in Aquatic Systems. J Aqua Pollut Toxicol. 9.36.
Copyright: © Figueiredo N, et al. This is an open-access article distributed under the terms of the Creative Commons
Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author
and source are credited.