Keywords
Checklist; Critical care; Catheter-related infection
Introduction
Malaria in pregnancy is one of the dreaded causes of fetal mortality and morbidity. High grades of fever, hypoglycemia, anemia and other complications can all adversely affect the fetus. Falciparum malaria can cause problems for the fetus with mortality up to 15-70% [1]. Spontaneous abortion, still birth, placental insufficiency and IUGR (temporary/chronic), low birth weight, fetal distress are the different problems observed in the growing fetus. Transplacental spread of malarial parasites results in congenital malaria [2]. In endemic areas, high prevalence of neonatal parasitemia has been reported, with majority of the parasitemic newborns being asymptomatic, however, the mortality was found to be higher in the parasitemic newborns compared with the aparasitemic and in the symptomatic compared with the asymptomatic [3]. Congenital malaria due to transplacental or perinatal infection of the fetus is being increasingly reported. It has been reported in 8-33% of pregnancies from both malaria-endemic and non-endemic areas [4]. It has been reported following maternal infections with all four species of human plasmodium, though most cases are reported following P. falciparum P. vivax. In non-endemic countries, P. malariae may cause a disproportionately higher number of congenital malaria cases due to its longer persistence in the host. In endemic areas symptomatic malaria in the neonate is rare despite a high incidence of maternal parasitemia and placental malaria as maternally derived IgG and the high proportion of fetal hemoglobin inhibit parasite development [5]. The symptoms of malaria during pregnancy differ with the intensity of malaria transmission and with the level of immunity acquired by the pregnant women [6].
This research work was designed to investigate the prevalence of malaria parasite among pregnant women who visit antenatal clinic at Mother and Child Specialist Hospital, Ondo, Nigeria. Their level of awareness, personal hygiene, use of various treatment such as anti-malaria drugs, herbs, use of insecticides and mosquito nets in preventing the transmission of the malaria parasites was assessed.
Materials and Methods
Study area and population
The study population consisted of 54 patients among pregnant women who came for antenatal at Mother and Child Specialist Hospital, Ondo, Ondo State, Nigeria between September and October, 2015. They were selected randomly without prior knowledge of their clinical and family history.
Sample collection
Blood samples were obtained from selected pregnant women using venous blood collection process. The collection was done by experts in the medical field. The blood samples were collected in EDTA bottles. The patients were swabbed with 70% alcohol and care was taken while using syringes Excessive bleeding was avoided.
Questionnaire
A structural questionnaire was used to elicit information from the patients. The questionnaire obtained information on: Age, Drugs Usage, Hygiene, Herbs Usage, Use of Mosquito Nets, Trimester Stages of the Pregnancy, Use of Insecticides. The questions in the questionnaires were asked from the patients and answers were given immediately. The answers to the questions were used for data analysis.
Rapid kit test method to detect plasmodium
Rapid Diagnostic Test (RDT), a lateral flow Immunochromatographic antigen-detection test was employed. It relied on the capture of dye-labeled antibodies to produce a visible band on a strip of nitro-cellulose. With malaria RDTs, the dye-labeled antibody first binds to a parasite antigen, and the resultant complex is captured on the strip by a band of bound antibodyforming a visible line (test line). A control line gives information on the integrity of the antibody-dye conjugate.
Microscopy
Microscopy, astandard for laboratory confirmation of malaria was employed. A drop of the patient’s blood was collected by finger prick, or from a larger venous blood specimen. It was spread on a glass slide (blood smear), dipped in a reagent that stains the malaria parasites (Giemsa stain) and then examined under a microscope at a 1000-fold magnification. Malaria parasites are recognizable by their physical features and by the appearance of the red blood cells that they infect. They can then be counted using the following method:
+ 1-10 asexual parasites per 100 thick film fields
++ 11-100 asexual parasites per 100 thick film fields
+++ 1-10 asexual parasites per single thick film field
++++ More than 10 asexual parasites per single thick film field
Data analysis
The data obtained from the information on the questionnaires were subjected to statistical analysis using statistical package (SPSS) to determine any significant relationship between: Age, Drugs usage, Hygiene, Herbs usage, Use of mosquito nets, Trimester stages of pregnancy, Use of insecticides.
Results
As observed in Table 1, out of the total 54 individual tested for Plasmodium falciparum using malaria kit, 2 (3.7%) were below 20 years, 39 (72.2%) were between 21 and 30 years, 12 (22.2%) were between 31 and 40 years and 1 (1.9%) were above 40 years.
| Malaria kit* age |
Age |
Total |
| <20 |
21-30 |
31-40 |
>40 |
| MP test |
Positive |
Count |
0 |
9 |
3 |
1 |
13 |
| % within MP test |
0.00% |
69.20% |
23.10% |
7.70% |
100.00% |
| % within age |
0.00% |
23.10% |
25.00% |
100.00% |
24.10% |
| Negative |
Count |
2 |
30 |
9 |
0 |
41 |
| % within MP test |
4.90% |
73.20% |
22.00% |
0.00% |
100.00% |
| % within age |
100.00% |
76.90% |
75.00% |
0.00% |
75.90% |
| Total |
Count |
2 |
39 |
12 |
1 |
54 |
| % within MP_test |
3.70% |
72.20% |
22.20% |
1.90% |
100.00% |
| % within age |
100.00% |
100.00% |
100.00% |
100.00% |
100.00% |
Table 1: The prevalence of malaria parasite among pregnant women tested for malaria using malaria parasite kit in relation to age of the women.
Out of the 2 (3.7%) that were below 20 years 0 (0 %) were positive, while 2 (4.9%) were mp negative. Out of the 39 (72.2%) that were between 21 and 30 years, 9 (69.2%) were mp positive while 30 (73.2%) were mp negative. For the 12 (22.2%) that were between 31 and 40 years, 3 (23.1%) were mp positive and 9 (22.0%) were mp negative. On the bar chart, for the positive results of the test for Plasmodium falciparum using malaria kit, women within the age group 21 and 30 years had the highest prevalence rate of 9 (23.1%) followed by women within the age group 31 and 40 years with prevalence rate of 3 (25.0%) (Figure 1). This was followed by women at age group greater than 40 years with prevalence rate of 1 (100.0%). Women at age group lesser than 20 years had the least prevalence rate of 0 (0.0%).
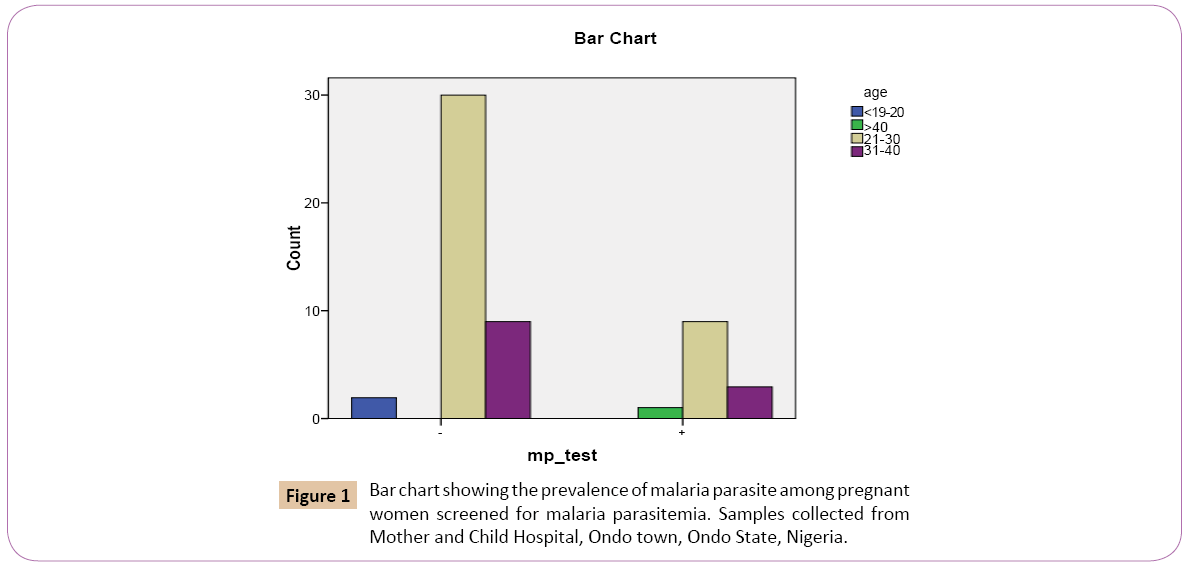
Figure 1: Bar chart showing the prevalence of malaria parasite among pregnant women screened for malaria parasitemia. Samples collected from Mother and Child Hospital, Ondo town, Ondo State, Nigeria.
Table 2 above shows the prevalence of malaria parasite in pregnant women and trimester stages of pregnancy. Out of the total 54 women that were tested for the presence of Plasmodium falciparum using malaria kit 3 (5.6%) were in the first trimester stage, 27 (50.0%) were in the second trimester stage, 24 (44.4%) were in the third trimester stage.
| |
Trimester stages |
Total |
| 1st stage |
2nd stage |
3rd stage |
| Mp test |
Positive |
Count |
0 |
6 |
7 |
13 |
| % within MP test |
0.00% |
46.20% |
53.80% |
100.00% |
| % within trimester stage |
0.00% |
22.20% |
29.20% |
24.10% |
| Negative |
Count |
3 |
21 |
17 |
41 |
| % within MP test |
7.30% |
51.20% |
41.50% |
100.00% |
| % within trimester stage |
100.00% |
77.80% |
70.80% |
75.90% |
| Total |
Count |
3 |
27 |
24 |
54 |
| % within MP test |
5.60% |
50.00% |
44.40% |
100.00% |
| % within trimester stage |
100.00% |
100.00% |
100.00% |
100.00% |
Table 2: The prevalence of malaria parasite at different trimester stages of pregnancy among women subject.
Out of the 3 (5.6%) that were in the first trimester stage, show no malaria parasitemia in their blood sample 3 (7.3%) show negative results. Out of the 27 (50%) that were in the second trimester stage, 6 (46.2%) were mp positive while 21 (51.2%) were mp negative, of 24 (44.4%) that were in the third trimester stage, 7 (53.8%) show mp positive, while 17 (41.5%) were mp negative (Figure 2).
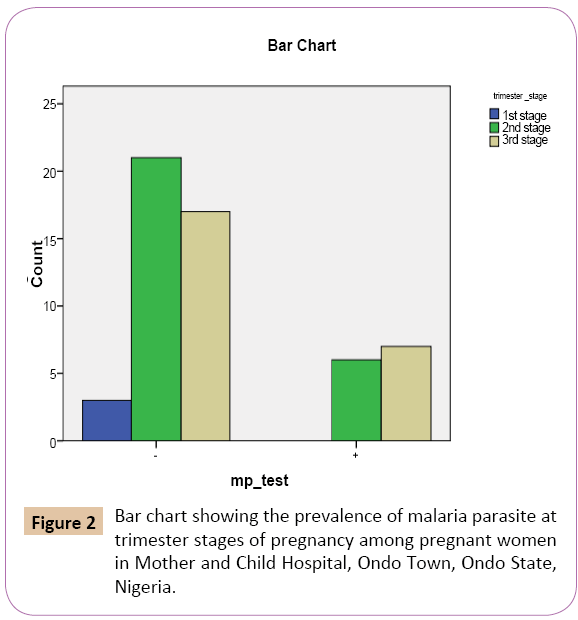
Figure 2: Bar chart showing the prevalence of malaria parasite at trimester stages of pregnancy among pregnant women in Mother and Child Hospital, Ondo Town, Ondo State, Nigeria.
On the bar chart (Figure 3) showing positive result of the test for malaria parasite using malaria kit, women in the third trimester stage has the highest prevalence rate of 7 (29.2%), followed by women in the second trimester stage with prevalence rate of 6 (22.2%). The women in the first trimester stage has the lowest prevalence rate of 0 (0.0%)
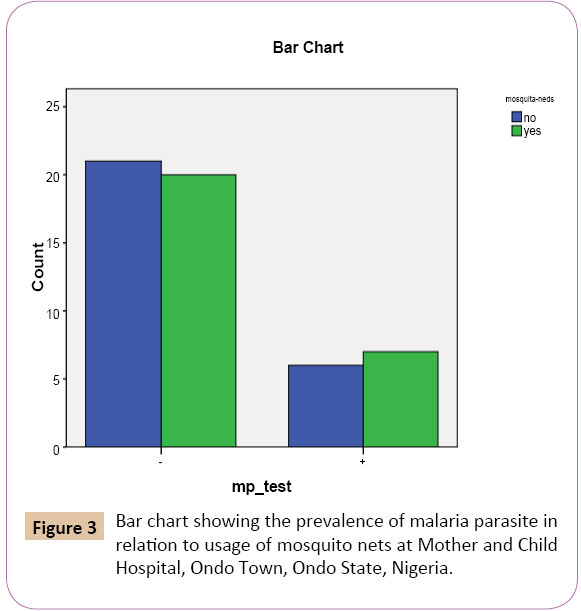
Figure 3: Bar chart showing the prevalence of malaria parasite in relation to usage of mosquito nets at Mother and Child Hospital, Ondo Town, Ondo State, Nigeria.
Table 3 above shows the prevalence of malaria parasite in pregnant women in relation to the use of mosquito nets. Out of the total 54 women that were tested for the presence of Plasmodium falciparum using malaria kit, 27 (50.0%) said they did not use mosquito nets, while 27 (50.0%) said they did use mosquito nets.
| Mosquito nets |
No |
Yes |
Total |
| Mp test |
Positive |
Count |
6 |
7 |
13 |
| % within MP test |
46.20% |
53.80% |
100.00% |
| % within mosquito nets |
22.20% |
25.90% |
24.10% |
| Negative |
Count |
21 |
20 |
41 |
| % within MP test |
51.20% |
48.80% |
100.00% |
| % within mosquito nets |
77.80% |
74.10% |
75.90% |
| Total |
Count |
27 |
27 |
54 |
| % within MP test |
50.00% |
50.00% |
100.00% |
| % within mosquito nets |
100.00% |
100.00% |
100.00% |
Table 3: The prevalence of malaria parasite in pregnant women in relation to usage of mosquito nets.
Out of the 27 (50.0%) that do not use mosquito nets, 6 (46.2%) were positive while 21 (51.2%) were negative. Of the 27 (50.0%) that do use mosquito nets 7 (53.8%) were mp positive while 20 (48.8%) were negative.
On the bar chart, for the positive results of the test for malaria parasite using malaria kit, women who do always use mosquito nets had the highest prevalence rate 7 (25.9%) while the women who do not use mosquito nets had the lowest prevalence rate 6 (22.2%).
As observed in Table 4 above, of the total 54 individual tested for Plasmodium falciparum using malaria kit, 41 (75.9%) denied usage of herbs for treatment for malaria while 13 (24.1%) confirmed the use of herbs as treatment for malaria.
| Malaria kit* herbs |
Herbs |
Total |
| NO |
YES |
| Mp test |
Positive |
Count |
8 |
5 |
13 |
| % within MP test |
61.50% |
38.50% |
100.00% |
| % within herbs |
19.50% |
38.50% |
24.10% |
| Negative |
Count |
33 |
8 |
41 |
| % within MP test |
80.50% |
19.50% |
100.00% |
| % within herbs |
80.50% |
61.50% |
75.90% |
| Total |
Count |
41 |
13 |
54 |
| % within MP test |
75.90% |
24.10% |
100.00% |
| % within herbs |
100.00% |
100.00% |
100.00% |
Table 4: The prevalence of malaria parasite among pregnant women tested using malaria Parasite kit in relation to their usage of herbs as treatment for malaria.
Out of the 41 (75.9%) that denied usage of herbs as treatment for malaria, 8 (61.5%) were positive, while 33 (80.5%) were negative. Out of 13 (24.1%) that confirmed the usage of herbs for malaria treatment, 5 (38.5%) were positive while 8 (19.5%) were negative (Figure 4).
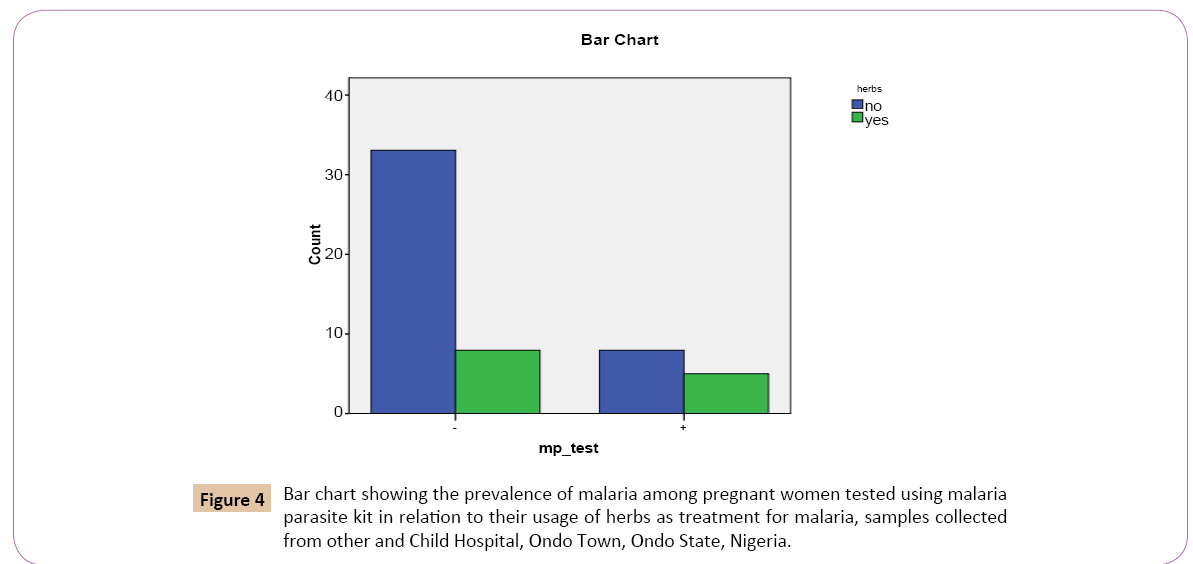
Figure 4: Bar chart showing the prevalence of malaria among pregnant women tested using malaria parasite kit in relation to their usage of herbs as treatment for malaria, samples collected from other and Child Hospital, Ondo Town, Ondo State, Nigeria.
On the bar chart, for the positive results of the test for Plasmodium falciparum using malaria kit, women who denied the use of herbs as malaria treatment had the highest prevalence rate of 8 (19.5%) while the women who confirmed the use of herbs as treatment for malaria treatment had the lowest prevalence rate 5 (38.5%).
Out of the total 54 individual tested for Plasmodium falciparum using malaria kit, 30 (55.6%) denied the use of drugs as treatment for malaria, 24 (44.4%) confirmed the use of drugs as treatment for malaria (Table 5).
| Malaria kit* drugs |
Drugs |
Total |
| No |
Yes |
| Mp test |
Positive |
Count |
7 |
6 |
13 |
| % within mp_test |
53.80% |
46.20% |
100.00% |
| % within drugs |
23.30% |
25.00% |
24.10% |
| Negative |
Count |
23 |
18 |
41 |
| % within mp_test |
56.10% |
43.90% |
100.00% |
| % within drugs |
76.70% |
75.00% |
75.90% |
| Total |
Count |
30 |
24 |
54 |
| % within mp_test |
55.60% |
44.40% |
100.00% |
| % within drugs |
100.00% |
100.00% |
100.00% |
Table 5: The prevalence of malaria parasite among pregnant women tested for malaria using malaria parasite kit in relation to their usage of drugs as treatment for malaria.
Out of the 30 (55.6%) that denied the use of drugs as treatment for malaria, 7 (53.8%) tested mp positive while 23 (56.1%) tested mp negative, Out of the 24 (44.4%) that confirmed the use of drugs as treatment for malaria, 6 (46.2%) tested positive while 18 (43.9%) tested negative (Table 5).
On the bar chart, for the positive results of the test for Plasmodium falciparum using malaria kit, women who denied the use of drugs as treatment for malaria had the highest prevalence rate 7 (23.3%). Women who confirmed the use of drugs as treatment of malaria had the lowest prevalence rate 6 (25.0%) (Figure 5).
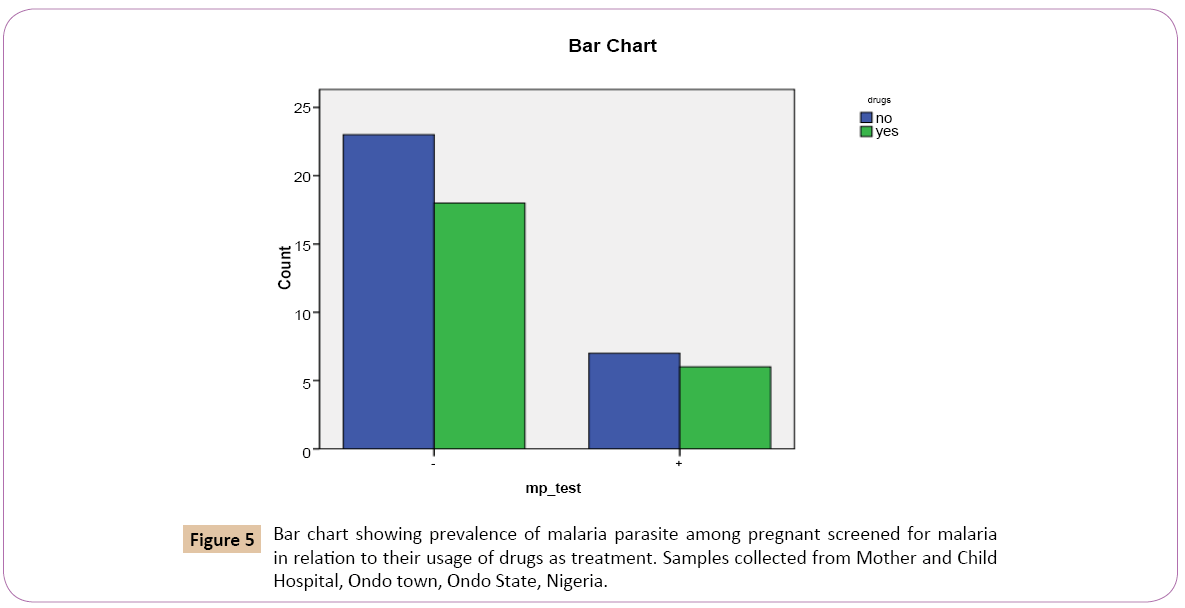
Figure 5: Bar chart showing prevalence of malaria parasite among pregnant screened for malaria in relation to their usage of drugs as treatment. Samples collected from Mother and Child Hospital, Ondo town, Ondo State, Nigeria.
Out of the total 54 individuals tested for Plasmodium falciparum using malaria kit, 28 (51.9%) denied the use of insecticides as preventive measures against malaria. 26 (48.1%) confirmed the use of insecticides as preventive measures against malaria (Table 6).
| Malaria kit* insecticide |
Insecticide |
Total |
| No |
Yes |
| Mp test |
Positive |
Count |
5 |
8 |
13 |
| % within MP test |
38.50% |
61.50% |
100.00% |
| % within insecticide |
17.90% |
30.80% |
24.10% |
| Negative |
Count |
23 |
18 |
41 |
| % within MP test |
56.10% |
43.90% |
100.00% |
| % within insecticide |
82.10% |
69.20% |
75.90% |
| Total |
Count |
28 |
26 |
54 |
| % within MP test |
51.90% |
48.10% |
100.00% |
| % within insecticide |
100.00% |
100.00% |
100.00% |
Table 6: The prevalence of malaria parasite in pregnant women tested for malaria using malaria parasite kit in relation to their usage of insecticides.
Out of 28 (51.9%) that denied the use of insecticides as preventive measures against malaria, 5 (38.5%) tested positive, 23 (56.1%) tested negative. Out of the 26 (48.1%) that confirmed the use of insecticides as preventive measures against malaria, 8 (61.5%) tested positive, 18 (43.9%) tested negative (Table 6).
On the bar chart, for the positive result of the test for Plasmodium falciparum using malaria kit, women who confirmed the use of insecticides as preventive measures against malaria had the highest rate 8 (30.8%) and women who denied the use of insecticide as preventive measures against malaria had the lowest prevalence rate 5 (17.9%) (Figure 6).
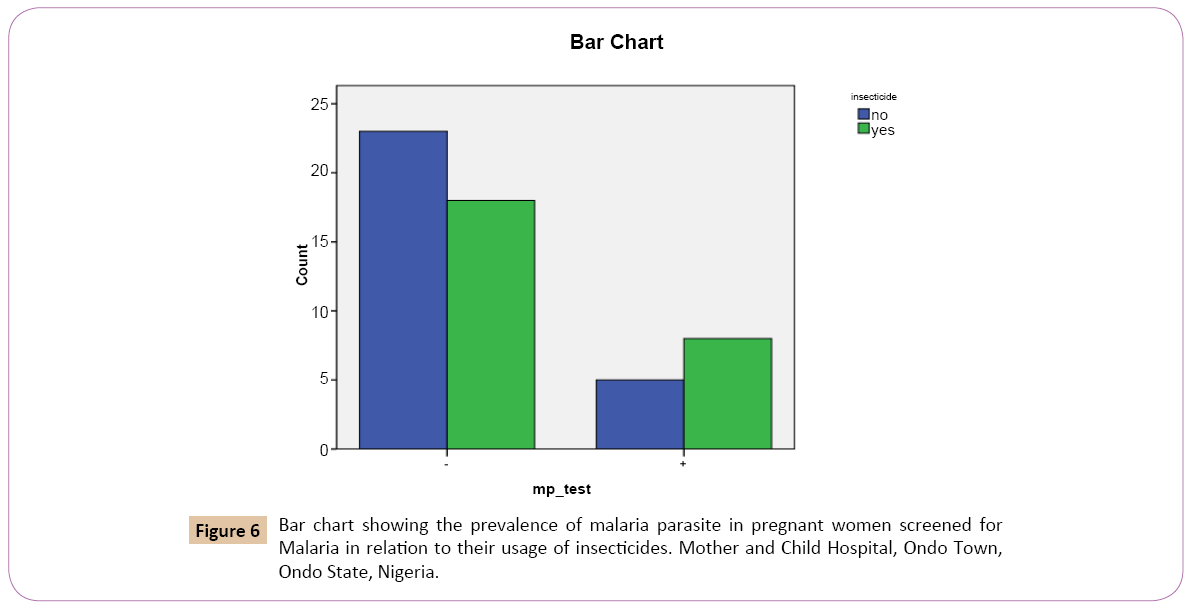
Figure 6: Bar chart showing the prevalence of malaria parasite in pregnant women screened for Malaria in relation to their usage of insecticides. Mother and Child Hospital, Ondo Town, Ondo State, Nigeria.
Out of the total 54 individuals (pregnant women) tested for Plasmodium falciparum using malaria kit, 26 (48.1%) had a clean environment, 10 (18.5%) had a dirty environment, 14 (25.9%) had mosquitoes in their environment, 4 (7.4%) kept their environment clean personally (Table 7).
| Malaria kit* hygiene |
Hygiene |
Total |
| Clean |
Dirty |
Mp present |
Well kept |
| MP test |
Positive |
Count |
4 |
3 |
6 |
0 |
13 |
| % within MP_test |
30.80% |
23.10% |
46.20% |
0.00% |
100.00% |
| % within hygiene |
15.40% |
30.00% |
42.90% |
0.00% |
24.10% |
| Negative |
Count |
22 |
7 |
8 |
4 |
41 |
| % within MP test |
53.70% |
17.10% |
19.50% |
9.80% |
100.00% |
| % within hygiene |
84.60% |
70.00% |
57.10% |
100.00% |
75.90% |
| Total |
Count |
26 |
10 |
14 |
4 |
54 |
| % within MP test |
48.10% |
18.50% |
25.90% |
7.40% |
100.00% |
| % within hygiene |
100.00% |
100.00% |
100.00% |
100.00% |
100.00% |
Table 7: The prevalence of malaria parasite in pregnant women tested for malaria using malaria parasite kit in relation to hygiene.
Out of the 26 (48.1%) pregnant women that had a clean environment, 4 (30.8%) tested positive while 22 (53.7%) tested negative. Out 10 (18.5%) that had a dirty environment 3 (23.1%) tested positive while 7 (17.1%) tested negative. Out of 14 (25.9%) women that has mosquitoes in their environment, 6 (46.2%) tested positive while 8 (19.5%) tested negative. Out of the 4 (7.4%) pregnant women that kept their environment clean personally 0 (0.0%) tested positive, 4 (9.8%) tested negative (Table 7).
On the bar chart, for the positive results of the test for the Plasmodium falciparum using malaria kit, women that had mosquitoes in their environment had the prevalence rate of 6 (42.9%) followed by women who had a clean environment with prevalence rate of 3 (30.0%). Women who personally kept their environment clean had the lowest prevalence rate of 0 (0.0%) (Figure 7).
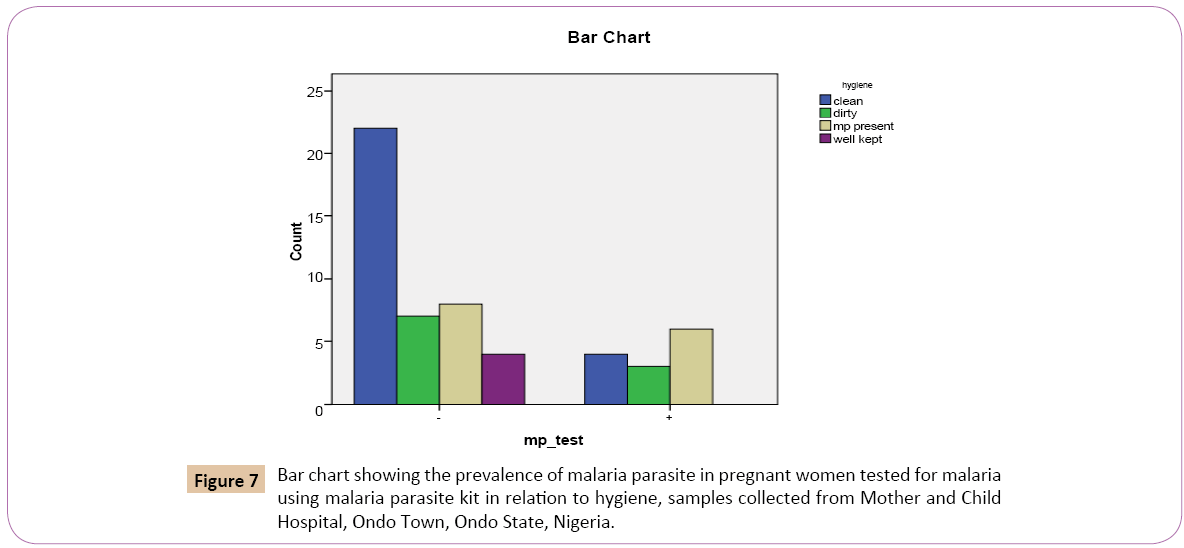
Figure 7: Bar chart showing the prevalence of malaria parasite in pregnant women tested for malaria using malaria parasite kit in relation to hygiene, samples collected from Mother and Child Hospital, Ondo Town, Ondo State, Nigeria.
Microscopic Analysis
According to Table 8 above, out of the total 54 individuals screened for Plasmodium falciparum using microscopic analysis. 2 (3.7%) were below 20 years, 39 (72.2%) were between 21 and 30 years, 2 (22.2%) were between 31 and 40 years and 1 (1.9%) was above 40 years.
| Microscopic analysis* age |
Age |
Total |
| 21-30 |
31-40 |
<20 |
>40 |
| Microscopic analysis |
+ |
Count |
8 |
3 |
0 |
0 |
11 |
| % within microscopic analysis |
72.70% |
27.30% |
0.00% |
0.00% |
100.00% |
| % within age |
20.50% |
25.00% |
0.00% |
0.00% |
20.40% |
| ++ |
Count |
15 |
2 |
1 |
0 |
18 |
| % within microscopic analysis |
83.30% |
11.10% |
5.60% |
0.00% |
100.00% |
| % within age |
38.50% |
16.70% |
50.00% |
0.00% |
33.30% |
| +++ |
Count |
11 |
4 |
0 |
1 |
16 |
| % within microscopic analysis |
68.80% |
25.00% |
0.00% |
6.20% |
100.00% |
| % within age |
28.20% |
33.30% |
0.00% |
100.00% |
29.60% |
| - |
Count |
5 |
3 |
1 |
0 |
9 |
| % within microscopic analysis |
55.60% |
33.30% |
11.10% |
0.00% |
100.00% |
| % within age |
12.80% |
25.00% |
50.00% |
0.00% |
16.70% |
| Total |
Count |
39 |
12 |
2 |
1 |
54 |
| % within microscopic analysis |
72.20% |
22.20% |
3.70% |
1.90% |
100.00% |
| % within age |
100.00% |
100.00% |
100.00% |
100.00% |
100.00% |
Table 8: The prevalence of malaria parasite among pregnant women tested for malaria using microscopic analysis in relation to age of the women.
Of the 2 (3.7%) that was below 20 years, 0 (0.0%) had low positive parasitaemia count (+). 1 (5.6%) had high positive parasitaemia count (++). 0 (0.0%) had very high positive parasitaemia count (+++) while 1 (11.1%) had negative parasitaemia count (-). Out of 39 (72.2%) that were between 21 and 30 years, 8 (72.7%) had low positive parasitaemia count (+), 15 (83.3%) had high positive parasitaemia count (++), 11 (68.8%) had very high positive parasitaemia count while 5 (55.6%) had negative parasitaemia count (-), Out of 12 (22.2%) that were between 31 and 40 years, 3 (27.3%) had low positive parasitaemia count (+), 2 (11.1%) had high positive parasitaemia count (++), 4 (25.0%) had very high positive parasitaemia count (+++) while 3 (33.3%) had negative parasitaemia count (-). Out of the 1 (1.9%) that were above 40 years, 0 (0.0%) had low positive parasitaemia count (+), 0 (0.0%) had high positive parasitaemia count (++), 1 (6.2%) had very high positive parasitaemia count (+++), while 0 (0.0%) had negative parasitaemia count (Figure 8).
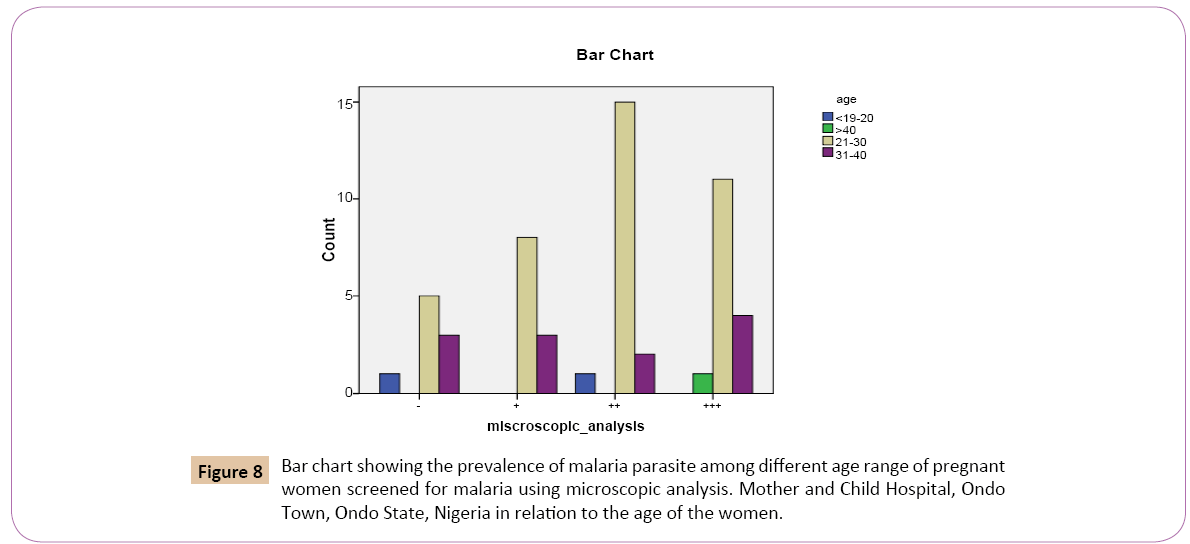
.
Figure 8: Bar chart showing the prevalence of malaria parasite among different age range of pregnant women screened for malaria using microscopic analysis. Mother and Child Hospital, Ondo Town, Ondo State, Nigeria in relation to the age of the women.
Out of the total 54 individual tested for Plasmodium falciparum using microscopic analysis. 3 (5.6%) were in the first trimester stage of pregnancy, 27 (50.0%) were in the second trimester stage, 24 (44.4%) were in the third trimester stage of pregnancy (Table 9).
| Microscopic analysis *trimester stage |
Trimester_stage |
Total |
| 1st stage |
2nd stage |
3rd stage |
| Microscopic analysis |
+ |
Count |
1 |
6 |
4 |
11 |
| % within microscopic analysis |
9.10% |
54.50% |
36.40% |
100.00% |
| % within trimester stage |
33.30% |
22.20% |
16.70% |
20.40% |
| ++ |
Count |
0 |
9 |
9 |
18 |
| % within microscopic analysis |
0.00% |
50.00% |
50.00% |
100.00% |
| % within trimester stage |
0.00% |
33.30% |
37.50% |
33.30% |
| +++ |
Count |
0 |
8 |
8 |
16 |
| % within microscopic analysis |
0.00% |
50.00% |
50.00% |
100.00% |
| % within trimester stage |
0.00% |
29.60% |
33.30% |
29.60% |
| - |
Count |
2 |
4 |
3 |
9 |
| % within microscopic analysis |
22.20% |
44.40% |
33.30% |
100.00% |
| % within trimester stage |
66.70% |
14.80% |
12.50% |
16.70% |
| Total |
Count |
3 |
27 |
24 |
54 |
| % within microscopic analysis |
5.60% |
50.00% |
44.40% |
100.00% |
| % within trimester stage |
100.00% |
100.00% |
100.00% |
100.00% |
Table 9: The prevalence of malaria parasite among pregnant women tested for malaria using microscopic analysisof blood samples in relation to trimester stage of pregnancy.
Out of the 3 (5.6%) that were in the first trimester stage of pregnancy, 1 (9.1%) had low positive parasitaemia count (+), 0 (0.0%) had high positive parasitaemia (++), 0 (0.0%) had very high positive parasitaemiacount (+++), while 2 (22.2%) had negative parasitaemia count (-). Out of the 27 (50.0%) that were in the second trimester stage, 6 (54.5%) had low positive parasitaemia (+), 9 (50.0%) had high positive parasitaemia count (++), 8 (50.0%) had very high positive parasitaemia count (+++), while 3 (33.3%) had negative parasitaemia count (Figure 9).
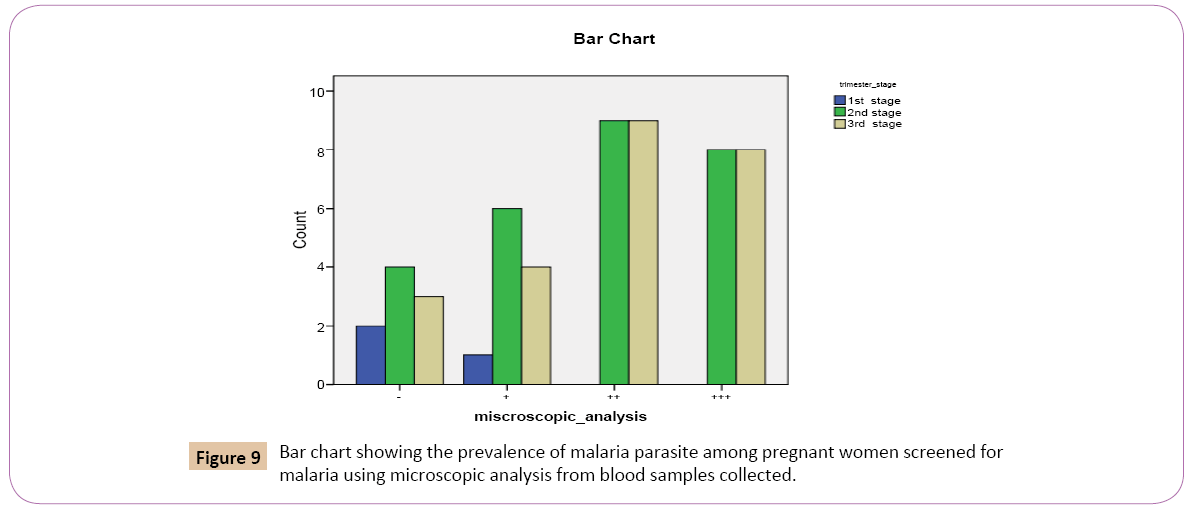
Figure 9: Bar chart showing the prevalence of malaria parasite among pregnant women screened for malaria using microscopic analysis from blood samples collected.
Out of the total 54 individuals tested for Plasmodium falciparum using microscopic analysis: 27 (50.0%) confirmed the use of mosquito nets, 27 (50.0%) denied the use of mosquito nets (Table 10). Out of the 27 (50.0%) that confirmed the use of mosquito nets, 6 (54.5%) had low positive parasitaemia count (+), 8 (44.4%) had high positive parasitaemia count (++), 7 (43.8%) had very high parasitaemiacount (+++), while 6 (66.7%) had negative parasitaemia count (-). Out of the 27 (50.0%) that denied the use of mosquito, 5 (45.5%) had low positive parasitaemia count (+), 10 (55.6%) had high positive parasitaemia count, 9 (56.2%) had very high positive parasitaemia count, while 3 (33.3%) had negative parasitaemia count (Figure 10).
| Microscopic analysis* mosquito nets |
Mosquito_nets |
Total |
| No |
Yes |
| Microscopic analysis |
+ |
Count |
5 |
6 |
11 |
| % within microscopic analysis |
45.50% |
54.50% |
100.00% |
| % within mosquito_nets |
18.50% |
22.20% |
20.40% |
| ++ |
Count |
10 |
8 |
18 |
| % within microscopic analysis |
55.60% |
44.40% |
100.00% |
| % within mosquito_nets |
37.00% |
29.60% |
33.30% |
| +++ |
Count |
9 |
7 |
16 |
| % within microscopic analysis |
56.20% |
43.80% |
100.00% |
| % within mosquito_nets |
33.30% |
25.90% |
29.60% |
| - |
Count |
3 |
6 |
9 |
| % within microscopic analysis |
33.30% |
66.70% |
100.00% |
| % within mosquito_nets |
11.10% |
22.20% |
16.70% |
| Total |
Count |
27 |
27 |
54 |
| % within microscopic analysis |
50.00% |
50.00% |
100.00% |
| % within mosquito_nets |
100.00% |
100.00% |
100.00% |
Table 10: The prevalence of malaria parasite among pregnant women screened for malaria using microscopic analysis of blood samples in relation to usage of mosquito nets.
From Table 11 above, out of the total 54 individuals tested for Plasmodium falciparum using microscopic analysis. 41 (75.9%) denied the use of herbs as treatment against malaria, 13 (24.1%) confirmed the use of herbs as treatment against malaria.
Out of the 41 (75.9%) that denied the use of herbs as treatment against malaria, 10 (90.9%) had low positive parasitaemia count (+), 15 (83.3%) had high positive parasitaemia count (++), 10 (62.5%) had very high positive parasitaemia count (+++), while 6 (66.7%) had negative parasitaemia count.
| Microscopic analysis * herbs |
Herbs |
Total |
| No |
Yes |
| Microscopic analysis |
+ |
Count |
10 |
1 |
11 |
| % within microscopic analysis |
90.90% |
9.10% |
100.00% |
| % within herbs |
24.40% |
7.70% |
20.40% |
| ++ |
Count |
15 |
3 |
18 |
| % within microscopic analysis |
83.30% |
16.70% |
100.00% |
| % within herbs |
36.60% |
23.10% |
33.30% |
| +++ |
Count |
10 |
6 |
16 |
| % within microscopic analysis |
62.50% |
37.50% |
100.00% |
| % within herbs |
24.40% |
46.20% |
29.60% |
| - |
Count |
6 |
3 |
9 |
| % within microscopic analysis |
66.70% |
33.30% |
100.00% |
| % within herbs |
14.60% |
23.10% |
16.70% |
| Total |
Count |
41 |
13 |
54 |
| % within microscopic analysis |
75.90% |
24.10% |
100.00% |
| % within herbs |
100.00% |
100.00% |
100.00% |
Table 11: The prevalence of malaria parasite among pregnant women tested for malaria based on their usage of herbs for treatment.
Out of the 13 (24.1%) that confirmed the use of the herbs as treatment against malaria, 1 (9.1%) had low positive parasitaemia count (+), 3 (16.7%) had high positive parasitaemia count (++), 6 (37.5%) had very high positive parasitaemia count (+++), while 3 (33.3%) had negative parasitaemia count (-) (Figure 11).
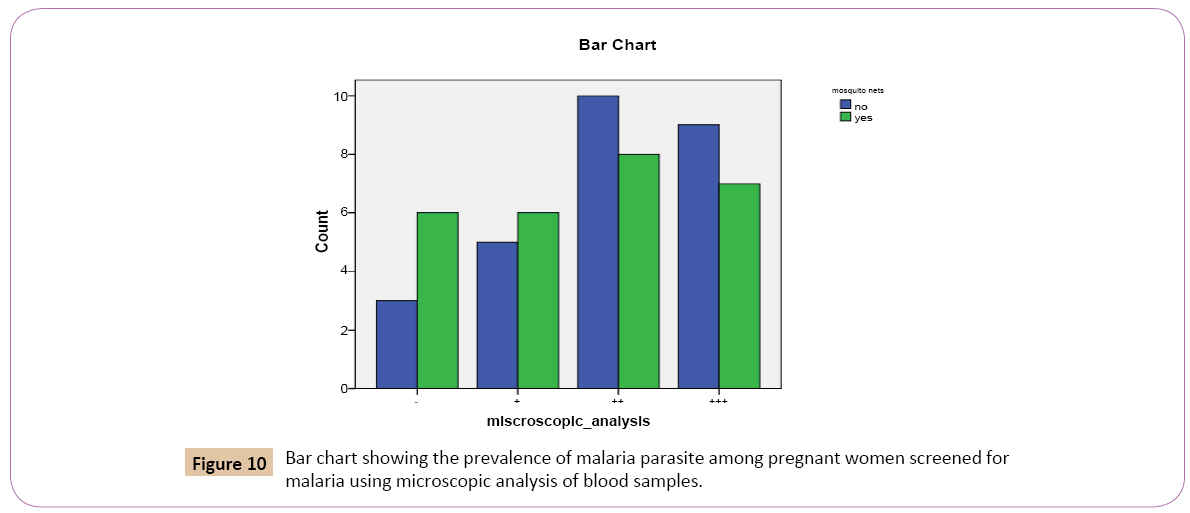
Figure 10: Bar chart showing the prevalence of malaria parasite among pregnant women screened for malaria using microscopic analysis of blood samples.
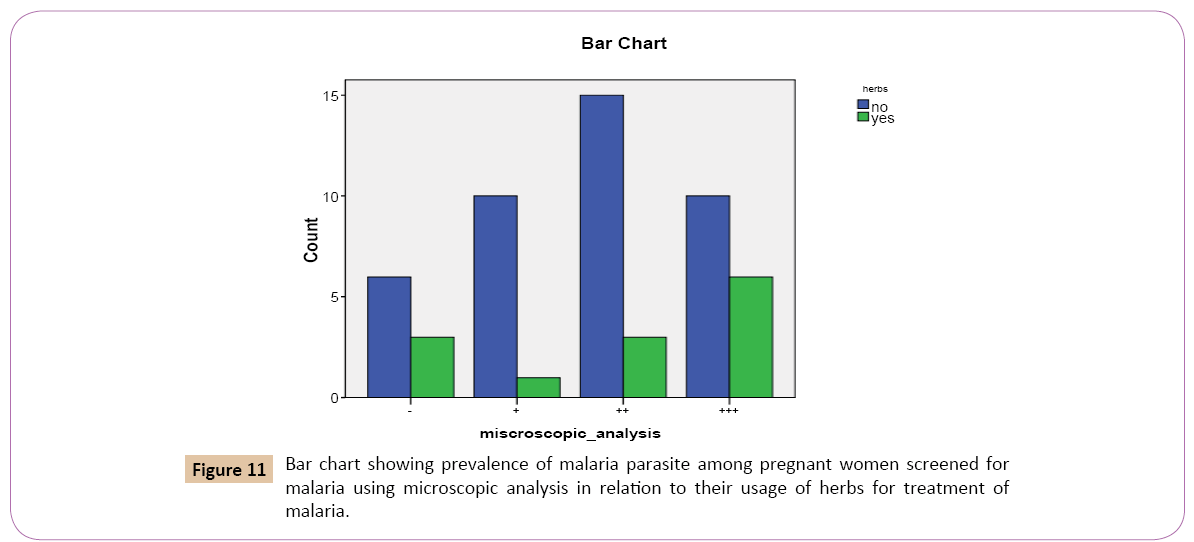
Figure 11: Bar chart showing prevalence of malaria parasite among pregnant women screened for malaria using microscopic analysis in relation to their usage of herbs for treatment of malaria.
From Table 12 above, out of the total 54 individual tested for Plasmodium falciparum using microscopic analysis. 30 (55.6%) denied the use of drugs as treatment against malaria, 24 (44.4%) confirmed the use of drugs as treatment against malaria.
| Microscopic analysis* drugs |
Drugs |
Total |
| No |
Yes |
| Microscopic analysis |
+ |
Count |
7 |
4 |
11 |
| % within microscopic analysis |
63.60% |
36.40% |
100.00% |
| % within drugs |
23.30% |
16.70% |
20.40% |
| ++ |
Count |
9 |
9 |
18 |
| % within microscopic analysis |
50.00% |
50.00% |
100.00% |
| % within drugs |
30.00% |
37.50% |
33.30% |
| +++ |
Count |
9 |
7 |
16 |
| % within microscopic analysis |
56.20% |
43.80% |
100.00% |
| % within drugs |
30.00% |
29.20% |
29.60% |
| - |
Count |
5 |
4 |
9 |
| % within microscopic analysis |
55.60% |
44.40% |
100.00% |
| % within drugs |
16.70% |
16.70% |
16.70% |
| Total |
Count |
30 |
24 |
54 |
| % within microscopic analysis |
55.60% |
44.40% |
100.00% |
| % within drugs |
100.00% |
100.00% |
100.00% |
Table 12: Screening analysis of blood samples in relation to their usage of drugs for treatment of malaria.
Out of the 30 (55.6%) that denied the use of drugs as treatment against malaria, 7 (63.6%) had low positive parasitaemia count (+), 9 (50.0%) had high positive parasitaemia count (++), 9 (56.2%) had very high positive parasitaemiacount (+++), while 5 (55.6%) had negative parasitaemia count (-). Out of the 24 (44.4%) that confirmed the use of drugs as treatments against malaria. 4 (36.4%) had low positive parasitaemia count (+), 9 (50.0%) had high positive parasitaemia count (++), 7 (43.8%) had very high positive parasitaemia count (+++) while 4 (44.4%) had negative parasitaemia count (-) (Figure 12).
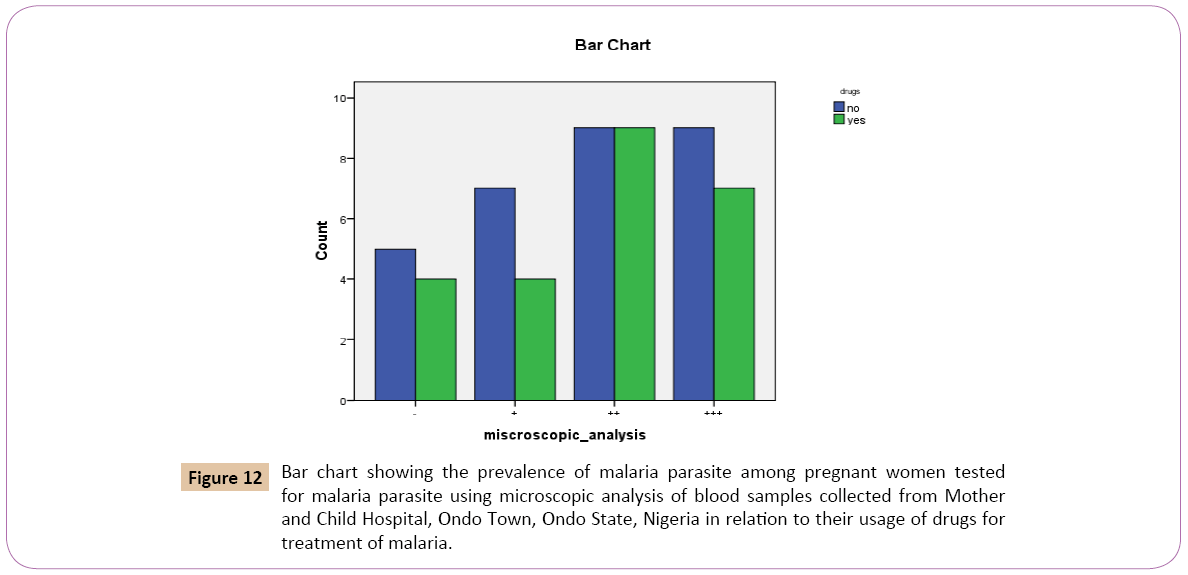
Figure 12: Bar chart showing the prevalence of malaria parasite among pregnant women tested for malaria parasite using microscopic analysis of blood samples collected from Mother and Child Hospital, Ondo Town, Ondo State, Nigeria in relation to their usage of drugs for treatment of malaria.
Out of the total 54 individuals tested for Plasmodium falciparum using microscopic analysis. 28 (51.9%) denied using insecticides to prevent malaria. 26 (48.1%) confirmed using insecticides to prevent malaria (Table 13).
Out of the 28 (51.9%) that denied using insecticides to prevent malaria, 5 (45.5%) had low positive parasitaemia count (+), 11 (61.1%) had high positive parasitaemia count (++), 8 (50.0%) had very high parasitaemiacount (+++), while 4 (44.4%) had negative parasitaemia count (Table 13).
| Microscopic analysis* insecticide |
Insecticide |
Total |
| No |
Yes |
| Microscopic analysis |
+ |
Count |
5 |
6 |
11 |
| % within microscopic analysis |
45.50% |
54.50% |
100.00% |
| % within insecticide |
17.90% |
23.10% |
20.40% |
| ++ |
Count |
11 |
7 |
18 |
| % within microscopic analysis |
61.10% |
38.90% |
100.00% |
| % within insecticide |
39.30% |
26.90% |
33.30% |
| +++ |
Count |
8 |
8 |
16 |
| % within microscopic analysis |
50.00% |
50.00% |
100.00% |
| % within insecticide |
28.60% |
30.80% |
29.60% |
| - |
Count |
4 |
5 |
9 |
| % within microscopic analysis |
44.40% |
55.60% |
100.00% |
| % within insecticide |
14.30% |
19.20% |
16.70% |
| Total |
Count |
28 |
26 |
54 |
| % within microscopic analysis |
51.90% |
48.10% |
100.00% |
| % within insecticide |
100.00% |
100.00% |
100.00% |
Table 13: The prevalence of malaria parasite among pregnant women tested for malaria parasite using microscopic analysis of blood samples in relation to their usage of insecticides.
Out of the 26 (48.1%) that confirmed using insecticides to prevent malaria, 6 (54.5%) had low positive parasitaemia count (+), 7 (38.9%) had high positive parasitaemia count (++), 8 (50.0%) had very high positive parasitaemia count (+++) while 5 (55.6%) had negative parasitaemia count (Figure 13).
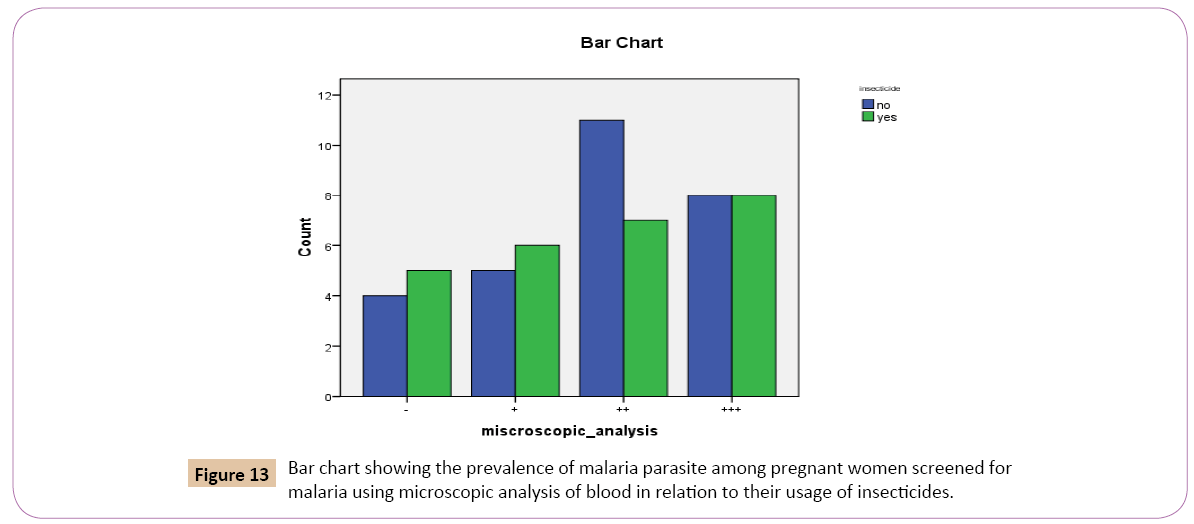
Figure 13: Bar chart showing the prevalence of malaria parasite among pregnant women screened for malaria using microscopic analysis of blood in relation to their usage of insecticides.
Discussion
The results obtained, from the laboratory diagnosis of the blood samples collected from the total 54 pregnant women screened using malaria rapid kit method and microscopic analysis showed that women within the age group 21 and 30 years had the highest prevalence rate compared to other age groups. Younger women seem to be more susceptible to malaria in this study area which contradicts the findings of Adefioye et al. who reported 36-39 age groups to be more susceptible [7]. Although this must have been due to their low immunity against malaria and number of child births they had had earlier because immunity increases with age and childbirths. However our result corresponds with the findings of Chimere et al. who reported that women within age group of 15-19 years had the highest prevalence, proving further that young maternal age increases the risk of malaria infection among patients [8]. Similar findings have been reported by other investigators in Gabon and Eastern Sudan where malaria prevalence was observed to decrease as age increased.
From the results of this study, women in the third trimester stage have shown the highest prevalence rate of 7 (29.2%) followed by women in the second trimester stage with prevalence rate of 6 (22.2%). The women in the first trimester stage had the lowest prevalence rate. This report is consistent with the findings of Thomas. et al. who reported that pregnant women in their third trimester stage were more infected than those at their first trimester because at the first trimester stage of pregnancy, women tend to protect themselves more and as the stages increases, their level of malaria prevention decreases, increasing the infection rates and this might also be due to the weak immunity of the women in the third trimester stage [9]. It is usually when they are very close to delivery that they become able to re-acquire their pre-pregnancy immunity. Also most of these pregnant women registered late for antenatal, which does not gives them opportunity to be well taken care of and given proper treatment and awareness, against the malaria infection and how to prevent it during pregnancy.
It can be deduced that women who always use mosquito nets seem to have the highest prevalence rate 7 (25.9%). The women who do not the mosquito nets had the prevalence rate 6 (22.2%). This corresponds with the findings of Ayaehieet al. who reported that there was a sustained but insignificant rise in asymptomatic malaria parasitaemia post-distribution of insecticide treatment nets [10]. Although the report survey at Enugu, Nigeria by Ugwuet al. proves that women who used insecticide treated nets are significantly less likely to have acute malaria, anaemia and babies with low birth weight than women who did not use insecticide treated nets. This result could have been due reasons such as the right use of mosquito nets, which might have required chemical washing, also some might have claimed to use these net sand might have not been using them effectively and constantly. Another reason might have been due to exposure to the vector (mosquitos) from the environment probably before sleeping under the nets. The mosquito nets will only be effective when the individuals are staying under them.
Women who denied the use of herbs had the highest prevalence rate, while the women who confirmed the use of herbs as treatment against malaria infections had the lowest prevalence rate. It commemorates the research work of Adefioye et al. in Osogbo, Nigeria reported that the use of herbs by pregnant women as both preventive and anti-malaria agents was 100% sensitive to Plasmodium falciparum infection [7]. In other words, the use of herbs as a cure against malaria is very effective although the Nigeria Federal Government needs to educate those involved in the practice of use of herbs as curatives or preventives on the normal dosage to be taken to avoid toxicological side effects. It is also advisable that the Food and Drug agencies of Nigeria should encourage more research into local herbs in order to develop new and more effective drug for prevention and control of malaria especially in the tropics where malaria is endemic.
Women who denied the use of drugs as control measures against malaria had the highest prevalence rate of infection while women who attested to the use of drugs had the lowest prevalence rate. This result further proves the effectiveness of chemoprophylaxis. This finding is consistent with reports from Ibadan and other African countries which found intermittent preventive treatment to be protective against malaria in pregnancy [8,11]. It has been reported that use the of un-recommended malaria chemo prophylactic medication such as chloroquine did not significantly reduce malaria infection among patients warning against wrong use of drugs [12]. Although prescription of drugs has a lot to do with the early detection of malaria parasite in pregnancy, once detected, effective drug usage depending on the trimester stage should be prescribed and administered immediately and should be well monitored to avoid complications. The use of drugs has been proven over the years to help in combating malaria and anaemia in pregnant women.
Women who confirmed the use of insecticides as effective in combating malaria infection had a higher prevalence rate than women who denied the use of insecticides. This contradicts investigations of Micheal et al. who reported in their study that 32.8% of 400 pregnant women used insecticide sprays with 2.3% of this population having the lowest malaria infection [13]. They suggested insecticide sprays as effective control measures against malaria. The findings of this research work might be due to the mutant and resilient nature of mosquitoes and also to the nature of the insecticide. Most insecticides are no longer as effective combating the new species of mosquitoes now prevalent in some parts of tropical Nigeria. It is suggestive and most preferable to use mosquito nets and probably effective insecticide creams which also serves as powerful agent in preventing mosquito bites. The use of insecticide spray is not a policy in Nigeria, but it is equally an effective malaria control strategy and if properly implemented, will impact positively on malaria control in Nigerian communities. As a recommendation it is advised that various uses of different classes of insecticides in conjunction with strict monitoring and public education should be strengthened in Nigeria.
Women in mosquito infested environments had the highest prevalence rate as compared the other prevailing environments. Mosquito infested environments pose a great deal of threat and risk to pregnant women. Women living in such environments stand 100% chances of contracting malaria parasite infections Environments such as stagnant pools, unkempt gutters, heaps of dirt, dirty and unused kitchens, keeping used water for long periods of time serve as factors promoting the breeding of different species of mosquitoes. This is consistent with the reports of Shr-jie et al. who in Ouagadougou, Burkina Faso found out that higher prevalence ratio of malaria occurred in areas where larvae breeding sites were semi-permanent [14]. Such sites include presence of dustbins, grasses, congesting, congestion, polluted gutter, stagnant waters and dirty surroundings covered with weeds in the rivers [15]. Therefore it is expedient to take good care of our environment and keep them well kept to avoid malaria parasite infection.
Conclusion
In conclusion, malaria in pregnant women is very threatening and could lead to high mortality rates. Therefore delivery of cost effective malaria prevention to pregnant women will require increased awareness of the problem among communities most affected with malaria. The use of insecticides, treated nets and drugs (chemoprophylaxis) may be beneficial to all women irrespective of their age or trimester stage during pregnancy. Also education and training programs in malaria prevention and early detection of malaria and treatment coupled with better health care delivery systems and enlightenment on the malaria transmission will be helpful [16].
It is also essential to avoid stagnant pools and poor environmental conditions which encourage the breeding and proliferation of mosquitoes. Much work needs to be done to educate the community and producer of herbs to strictly adhere to environmental hygiene since herbs are also effective in the prevention and cure of malaria.
Study Design Limitation
Based on our available procedures and constraint on Study Population at the center, the findings may not be translated to outcome of malaria parasitaemia associated with pregnant women of other Health Care Centers in the Country. However, the data obtained is of useful findings widely applicable as they will assist in control measures and effective therapeutic approach to malaria in pregnancy among women in all part of the world.
Acknowledgement
Authors would like to thank the Medical Personnel at Mother and Child Hospital Ondo, Nigeria for their immense support in the research samples collection in the Centre.
Conflict of Interest
Authors have no conflict of interest.
References
- Ribera JM, Hausmann-Muela S, D’Alessandro U, Grietens KP (2007) Malaria in pregnancy. What can the Social Sciences contribute. PLOS Med 4: 109-137.
- Bernard JB, Marian W, Ulrika U, Stephanie D, Jenny H, et al. (2008) Monitoring and evaluation of malaria in pregnancy- developing a rational basis for control. Malar J 7: S6.
- Adeola A, Catherine O, Henrientta U, Olugbenga AM, Aisha IM, et al. (2008) Clinical and laboratory features of congenital malaria in Nigeria. J Pediatric Infect Dis 3: 181-187.
- Valecha N, Bhatia S, Mehta S, Biswas S, Dash A (2007) Congenital malaria with a typical presentation, A case report from low transmission area in India. Malar J 6: 43.
- Gitau GM, John ME (2005) Malaria in pregnancy; Clinical, therapeutic and prophylactic consideration. Obstet Gynaecol 7: 5-11.
- WHO (World Health Organisation (2003) WHO/live at risk: Malaria in pregnancy.
- Adefioye OA, Adeyeba OA, Hassan W Oyeniran OA (2007) Prevalence of malaria parasite infection among pregnant women in Osogbo, Southwest, Nigerian. American-Eurasian Journal of Scientific Research 2: 43-45.
- Chimere OA, Wellington AO (2013) Factors associated with risk of malaria infection among pregnant women in Lagos, Nigeria. Infect Dis Poverty 2: 19.
- Hile T, Assam JP, Amali E, Amata E (2012) The Epidemiology of malaria among pregnant women in Garoua, Northern Cameroon. Journal of Parasitology and Vector Biology: 1-5.
- Anyaehie U, Nwagha UI, Aniebue PN, Nwagha TU (2011) The effect of free distribution of insecticide- treated nets on asymptomatic Plasmodium parasitaemia in pregnant and nursing mothers in a rural Nigerian community. Niger J Clin Pract 14: 19-22.
- Falade CO, Yusuf BO, Fadero FF, Mokuolu OA, Hamer DH, et al. (2007) Intermittent preventive treatment with sulphadoxine-pyrimethanoxine is effective in preventing maternal and placental malaria in Ibadan, south-western Nigeria. Malar J 6: 88.
- Ogungbamigbe TO, Ojurongbe O, Ogunro PS, Okanlawon BM, Kolawole SO (2008) Chloroquine resistant Plasmodium falciparum in Osogbo Nigeria, efficacy of amodiaquine+sulfadoxinepyrimethamine and chloroquine+chlorpheniramine for treatment. Meninst Oswaldo Cruz 103: 79-84.
- Micheal EF, Amoo AO, Akintunde GB, Oluwole OO, Konig W, et al. (2011) Use and Effects of malaria control measures in pregnancy in lagos, Nigeria. Korean J Parasitol 49: 365-371.
- Shr-jie W, Christian L, Thomas AS, Penelope V, Diallo AD, et al. (2006) Rapid urban malaria appraisal epidemiology of Urban. Malar J 4: 43.
- Idowu O (2014) Effect of environmental hygiene and water storage on the prevalence of malaria among pregnant women in Abeokuta Nigeria. Health 6: 90-93.
- Menendez C (1995) Malaria during pregnancy a priority area of malaria research and control. Parasitol Today 11: 178-183.