Stefan Stubinger1,2*, Ramon Bucher1, Peter Kronen2,3, Falko Schlottig4,5 and Brigitte von Rechenberg1,2
Hightech Research Center of Cranio- Maxillofacial Surgery, University of Basel, Gewerbestrasse 14-16, 4123 Allschwil, Switzerland
Center of Applied Biotechnology and Molecular Medicine (CABMM), Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland
Veterinary Anaesthesia Services, International (VAS), Z?rcherstrasse 39, CH-8400 Winterthur, Switzerland
Thommen Medical AG, Neckarsulmstrasse 28, 2540-Grenchen, Switzerland
Hochschule für Life Sciences, Gr?ndenstrasse 40, 4132 Muttenz, Switzerland
*Corresponding Author:
Stefan Stubinger
Center of Applied Biotechnology and Molecular Medicine (CABMM)
Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 190
CH-8057 Zurich
Switzerland
Tel: +4144635 8745
Fax: +41-44-635 8917
E-mail: stefan.stuebinger@cabmm.uzh.ch
Received date: February 15, 2016; Accepted date: February 19, 2016; Published date: February 22, 2016
Citation: Stubinger S, Bucher R, Kronen P, et al. Ligature-Induced Peri-Implantitis in Minipigs Revisited. 2016, 2:1.
Keywords
Mucositis; Bone loss; Inflammation; Dental implant; Animal model
Introduction
Treatment with osseointegrated dental implants has become a predictable and sustainable therapy for functional and esthetical reconstruction after tooth loss [1]. Yet, even though osseointegration of dental implants has clinically, anatomically, histologically, and ultrastructurally proven successful on longterm results inflammatory soft tissue issues, and there, mainly peri-implant mucositis and peri-implantitis are emerging problems [2-4]. In their consenus report Lindhe et al. concluded that peri-implant mucositis occurred in 80% of subjects and in 50% of implant sites, whereas peri-implantitis was identified in 28% and >56% of subjects and in 12% and 43% of implant sites [5]. This renders peri-implantitis a true threat in today’s advancement of implant reliability and performance.
In assessing the pathogenesis of peri-implant diseases, experimentally ligature-induced peri-implantitis lesions in animals have demonstrated histopathological similarities to naturally occurring lesions in humans [6]. Even though this chronic-type defect model using peri-implant ligatures was introduced in different large animal models, canines have established themselves as the most preferred species in the last decades. Schwarz et al. see the most distinct benefit of applying the canine model in the comfortable manageability by facilitating postoperative oral hygiene [7]. Furthermore, as anatomical dimensions of jawbones allow for placing conventional implants the authors regard canines as “[…] the most suitable animal species to conduct the ligature-induced peri-implantitis defect model […]”.
According to Martini et al. however, the known preference of applying dogs in research was mainly based on the fact that knowledge, scientific findings and instrumentation from veterinary therapeutic interventions could be easily transferred to the needs of in vivo testing [8]. This finally led to the widespread use and acceptance of this animal species, even if other large animal models were available and the use of companion animals was highly controversial. Being in agreement with this perception it was the aim of the present in-vivo trail to revisit the ligatureinduced peri-implantitis minipig model regarding its current scientific value and ethical justification in implant research [9]. The H1 hypothesis of this experimental study in mini pig was that ligature-induced peri-implantitis causes a hard tissue breakdown at the buccal site. The stated null hypothesis (H0) was that there are no differences in pocket depth (PD) and bleeding on probing (BOP) between healthy and infected sites.
Materials and Methods
Animals and surgical model
Six neutered male minipigs (Ellegaard Göttingen Minipigs A/S, Dalmose, Denmark) were used for this study (age: 1.4 ± 0.0 years; weight: 34.25 ± 1.6 kg). Animals were randomly allocated to an experimental silk ligature-induced peri-implantitis group (n=4 animals) and a reference healthy group (n=2 animals). After extraction of the premolars and a healing period of 8 weeks, each n= 4 dental implants (SPI® Element Inicell®, PF 4.0, length 8.0 mm, Thommen Medical AG, Grenchen, Switzerland) were placed in each mandible. After six weeks animals were sacrificed. All experiments were conducted according to the Swiss laws of animal protection and welfare and were authorized by the local federal authorities (authorization #73/2013).
Animal care
Minipigs were acclimatized at least 30 days prior to surgeries to the new husbandry, bacterial environment and feeding. Clinically, a veterinarian examined animals 1-2 days after arrival and before surgeries. Twice daily, animals were scored (alertness, posture, appetite, respiration, signs of pain, lameness, temperature) by a veterinarian and a trained veterinary technician. In patient records were kept for each animal beginning the day of arrival documenting daily observations, treatments, surgical protocols and postoperative recovery. If abnormal findings were found, a senior veterinarian was immediately notified. Only healthy animals without any visible signs of illness were used.
Upon arrival, feed was transitioned during a 3 week period from the supplier’s feed to pre-soaked, soft feed (600 g per animal per day, fed twice daily; KLIBA NAFAG 3000, Provimi Kliba AG, Kaiseraugst, Switzerland). Apple slices, apple sauce or yoghurt was fed by hand daily to tame the animals and was also used to give oral medication postoperatively. Enrichment included straw as bedding, toys, as well as a chain and wood to chew on. Even though animals were neutered, they showed excessive dominant behaviour when kept in groups of two or three. Animals were therefore kept solitary during the duration of the study but with visual contact to each other. Animals were regularly weighed at 2-4 week intervals. After surgery, enrichment was reduced to straw as bedding.
Anaesthesia and perioperative management
The day prior to surgery the animal was fed normally, bedding was removed overnight and feed was withheld preoperatively. Water was available ad libitum. After deep sedation in the stable (ketamine 20-40 mg/kg BW, Ketanarkon, Streuli Pharma AG, Uznach, Switzerland; midazolam 0.2 mg/kg BW; im, Sintetica S.A., Mendrisio, Switzerland), the animal was transported to surgery, an intravenous catheter was placed in an auricular vein, anesthesia was induced (propofol, to effect; Propofol® 1% Fresenius, Fresenius Kabi AG, Stans, Switzerland) and the animal was intubated in sternal recumbency. Carprofen (2 mg/ kg BW iv; Rimadyl®, Pfizer AG, Zurich, Switzerland), penicillin (10'000 IU/kg BW iv; Penicillin Natrium Streuli®, Streuli Pharma AG, Uznach, Switzerland) and methadone (2 mg/kg BW iv; Methadon Streuli®, Streuli Pharma AG, Uznach, Switzerland) were given perioperatively. Right before surgery of each side, the respective mandibular nerve was locally anesthetized (ropivacaine, to effect; NAROPIN Inj Lös 0.5%, AstraZeneca AG, Zug, Switzerland) at its entry side into the mandible. The effect was controlled using a peripheral nerve stimulator (Innervator®, Fisher and Paykel Healthcare, Melbourne, Australia). Adrenaline in a sterile solution (adrenaline, diluted to 1:200'000; ADRENALIN Amino Inj Lös, Amino AG, Neuhof, Switzerland) was injected in the local mucosa to reduce bleeding. Intraoperatively monitored parameters included electrocardiogram, heart rate, pulse rate, arterial blood pressure (ventral tail artery) and oxygen saturation. Anesthesia was maintained via inhalation anesthesia (isoflurane in oxygen; Attane Isoflurane, MINRAD INC., Buffalo, NY, USA) and a CRI of propofol for a balanced protocol. Body temperature was supported through a heating mattress and/or a ventilation system. Blood was taken for routine hematology and blood chemistry analysis to provide a foundation for treatment in case of postoperative complications or prolonged recovery.
After surgery, animals were transported back to the stable accompanied by a veterinarian and closely observed until full recovery. Animals were covered during postoperative transportation and recovery to decrease loss of heat due to convection. Heating lamps and ventilation systems were available in the stable in case of hypothermia. Surgical sites were treated with cooling packs to reduce swelling. The IV catheter was removed the day after the surgery.
Pain medication (methadone 1-4 mg/kg BW iv; or buprenorphine 0.015 mg/kg BW im or iv; Temgesic®, Reckitt Benckiser AG, Wallisellen, Switzerland) was given postoperatively, antibiotics (amoxicillin 500 mg, Synulox®, Pfizer AG, Zurich, Switzerland) and anti-inflammatory drugs (carprofen 4 mg/kg BW po) were given for 5 days.
Tooth extraction
The animals were placed in lateral recumbency and the mouth was kept open with a mouth gag. The head was positioned using a moldable surgery cushion. Adhering to the surgical principle of adequate access, sulcular incisions were performed around the premolars. Additionally, a slight mesial vertical releasing incision was performed to allow a careful elevation of a full thickness flap. Standard dental instruments (forceps, elevators) were used to loosen and extract teeth. Crowns of the molars were vertically and horizontally separated (iChiropro, Bien-Air Dental SA, Biel, Switzerland). Root remnants were either removed with special root elevators or were drilled out with the same dental drill under adequate irrigation. Extraction sockets were cleaned. The lingual soft tissue was loosened from the bone plate and the mucoperiosteal flap was gently retracted with an elevator. Afterwards, the alveolar bone crest was down-leveled for 1 to 2 mm and sharp bony edges were smoothened.
The buccal and lingual mucoperiosteal flaps were repositioned and closed using single sutures (Vicryl® 2/0, Ethicon, New Jersey, NY, USA). The animal was then turned to the other side and the surgical procedure was repeated in an identical manner on the other side of the mandible.
Implant placement
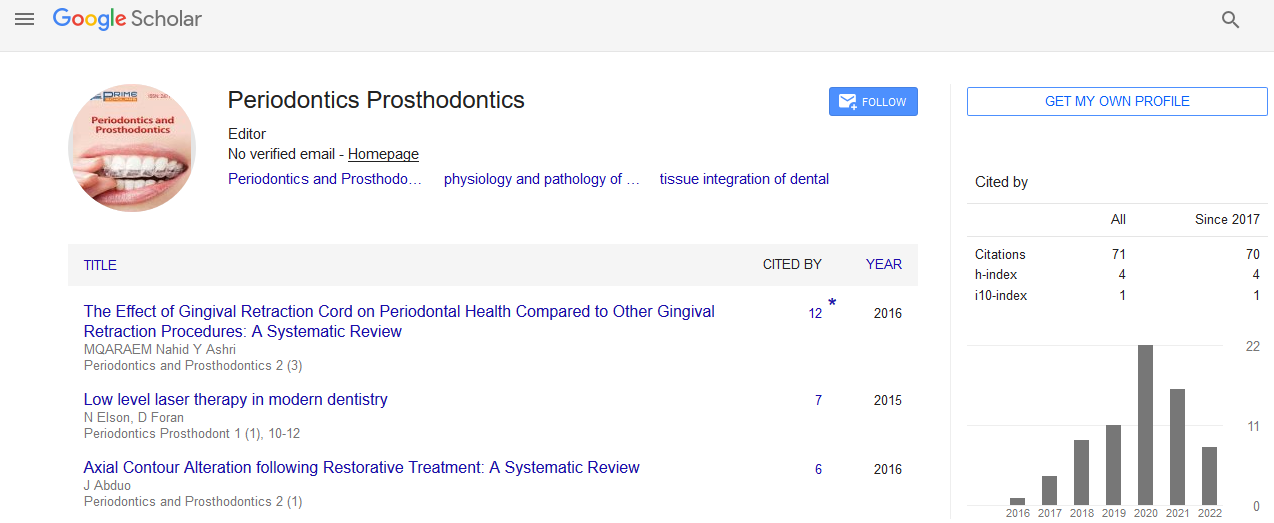
Following eight weeks of healing the anaesthetized mini pigs were placed analogous to the procedure of the first stage surgery. The alveolar ridge was accessed through a full-thickness flap using a slightly lingual, mid-crestal incision in combination with slightly curved vertical releasing-incisions at its mesial and distal end. The flap was then elevated and held back using special retraction hooks. Implant sites were prepared according to a standard and approved drilling protocol using rotating pilot and twist drills in ascending order (diameter). After careful removal of bone debris from the drill holes with sterile 0.9% physiological saline, implants (Figure 1) were inserted automatically (iChiropro, Bien-Air Dental S.A, Bienne, Switzerland). After gingival former fixation (PF 4.0, height 3.2 mm, Thommen Medical AG, Grenchen, Switzerland), mucoperiosteal flaps were repositioned and closed tension-free with single sutures (Vicryl® 2/0, Ethicon, New Jersey, NY, USA).
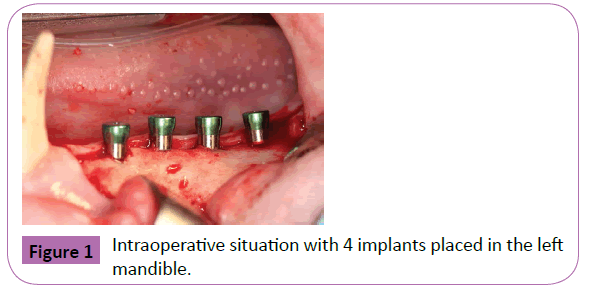
Figure 1: Intraoperative situation with 4 implants placed in the left mandible.
In the four animals of the experimental group, a single silk ligature (4.0) was placed around the abutment and slightly pushed downwards into the pocket. This finally led to a monolayer partly “submucosal” application. Ligatures were checked and maintained after two and four weeks (Figure 2). To reduce the burden of the animals and follow the 3R principles PD and BOP measurements were only performed at 6 weeks (time of sacrifice). BOP was evaluated as present if bleeding was evident within 10 s after probing, or absent, if no bleeding was noticed within 10 s after probing. PD was measured from the mucosal margin to the bottom of the probeable pocket. Each two measurements (mesial and distal) were performed at the lingual and buccal site. The statistical analysis was performed using a commercially available software program (SPSS® 18.0, SPSS Inc.). Mean values of all parameters were calculated.
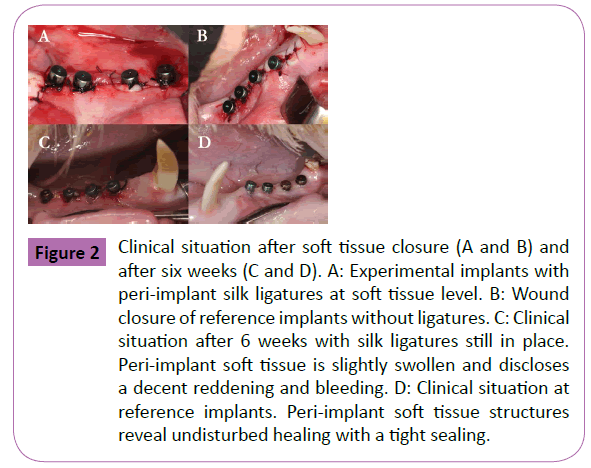
Figure 2: Clinical situation after soft tissue closure (A and B) and after six weeks (C and D). A: Experimental implants with peri-implant silk ligatures at soft tissue level. B: Wound closure of reference implants without ligatures. C: Clinical situation after 6 weeks with silk ligatures still in place. Peri-implant soft tissue is slightly swollen and discloses a decent reddening and bleeding. D: Clinical situation at reference implants. Peri-implant soft tissue structures reveal undisturbed healing with a tight sealing.
Results
In the present study ligature-induced peri-implantitis provoked a local inflammation and loss of crestal bone surrounding the implants in four out of four animals. Thereby, probing depth, and the presence of bleeding characterized a soft tissue breakdown, whereas microradiographs and histological sections confirmed a peri-implant bone loss with vestibular dehiscence defects (Figures 3 and 4). For healthy animals the mean pocket depths (PD) was 2.2 ± 1.1 mm. In contrast mean pocket depths for peri-implantitis sites were 5.4 ± 1.9 mm. Healthy sites showed no bleeding within 10 s after probing (60%) and a slight bleeding in 40% of the cases. In contrast 90% of peri-implantitis sites disclosed a severe bleeding within 10 s after probing. Only 10% of sites showed no bleeding after 10 s. Clinically, bleeding on probing and a pocket depth of more than 5 mm demonstrated a diseased status after 6 weeks in the ligature-induced peri-implantitis animals. Furthermore, the peri-implant gingiva was slightly swollen and displayed an intensive reddening. In contrast healthy animals showed some plaque formation and food remnants around the implants respectively the gingival formers. Yet bleeding was less conspicuous and peri-implant gingiva was less affected.
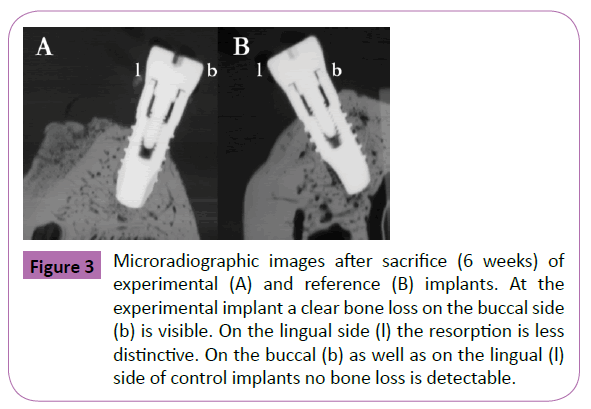
Figure 3: Microradiographic images after sacrifice (6 weeks) of experimental (A) and reference (B) implants. At the experimental implant a clear bone loss on the buccal side (b) is visible. On the lingual side (l) the resorption is less distinctive. On the buccal (b) as well as on the lingual (l) side of control implants no bone loss is detectable.
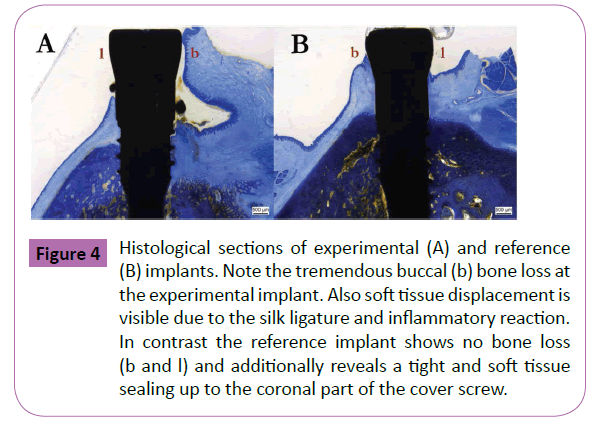
Figure 4: Histological sections of experimental (A) and reference (B) implants. Note the tremendous buccal (b) bone loss at the experimental implant. Also soft tissue displacement is visible due to the silk ligature and inflammatory reaction. In contrast the reference implant shows no bone loss (b and l) and additionally reveals a tight and soft tissue sealing up to the coronal part of the cover screw.
Overall, decrease of bone height was more prominent on the buccal aspect in all animals of the experimental group. Histologically, the measurable loss was up to three implant threads from bone level. On the lingual aspect the bony atrophy in the vertical direction was less distinctive with a mean bone loss of one implant thread. In half of the cases a formation of a bony pocket respectively crater could be detected. Mostly, however, resorption was not limited to the outer diameter of the silk ligature, but was more comprehensive. Soft tissue structures disclosed a pronounced detachment in all animals of the periimplantitis group, where upon healthy minipigs revealed a tight sealing along the whole implant collar and gingival former (2.5 mm ± 3.2 mm).
Discussion
Until today, the ligature-induced defect model still remains the model of first choice to experimentally investigate the cause, effect and treatment approaches of peri-implantitis [10]. By using this model, however, Alhag et al. reported that several factors such as clot adhesion/stability and cellular migration/ differentiation have to be carefully considered in the healing capacity of the peri-implant defect [11]. Therefore, Kolonidis et al. introduced an alternative model in Labrador dogs [12]. The authors placed titanium implants supracrestally allowing dental plaque to accumulate on exposed implant threads. After 5 weeks implants were cleaned and placed into fresh implant osteotomies on the contralateral side of the mandibles. Principally, this approach was based on the known perception that most dogs have a general tendency to develop a periodontal disease [13]. Even though the model was scientifically targeting to the right direction, burden and pain for the animals were ethically quite worth discussing. Beneath the much faster bone remodelling in dogs profound ethical constraints made this model do not become widely accepted. As similar emotional and legal issues count for the use of non-human primates in peri-implantitis research, their use is not well established in the European Community [14].
Generally, requirements and selection of a suitable animal model for peri-implantits include the necessity to investigate the pathogenesis of the disease as well as the host`s potential and degree of regeneration. Concerning overall bone composition and bone remodeling, minipigs reveal a close similarity to humans and thus offer interesting aspects for analyzing osseointegration in dental and craniofacial research [15-17]. Microbiologically, Hickey et al. could also demonstrate that artificially induced periimplantitis leads to a shift in the sulcular flora from primarily Gram-positive in healthy animals to Gram-negative in diseased animals [9]. Importantly, healthy animals do not disclose any specific species being characteristic for peri-implant lesions [7].
In conclusion, clinical, radiological and histological findings of the present animal experiment support the overall applicability of the ligature-induced peri-implantitis minipig model. The animals showed a progressive inflammatory soft tissue reaction with subsequent rapid breakdown of especially buccal peri-implant hard tissues. Operative prerequisites for generating the periimplant lesions were silk ligatures and neglect of any plaque control. With respect to anatomical, physiological and ethical issues this model also proved to be a reliable and justifiable animal model. Further experiments have to elaborate and document the microbiologic and molecular pathways in this model for an improved insight into the natural development of the disease and thereby allowing a plausible translation to the human situation.
Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007- 2013) under grant agreement n° [314911]. The authors thank our histology technicians Käthi Kämpf, Ladina Ettinger, Katalin Zlinszky, Sabina Wunderlin as well as Jola Plihal, for their outstanding histology specimens.
References
- Pjetursson BE, Sailer I, Zwahlen M, H?mmerle CH (2007) A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part I: single crowns. Clinical Oral Implants Research 18: 73-85.
- Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC (2009) Biology of implant osseointegration. Journal of Musculoskeletal and Neuronal Interactions9: 61-71.
- Vignoletti F, Abrahamsson I (2012)Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. Journal of Clinical Periodontology 12: 6-27.
- Heitz-Mayfield LJ(2008) Peri-implant diseases: diagnosis and risk indicators. Journal of Clinical Periodontology 35: 292-304.
- Lindhe J, Meyle J, Group D of European Workshop on Periodontology (2008) Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. Journal of Clinical Periodontology 35: 282-285.
- Berglundh T, Zitzmann NU, Donati M (2011) Are peri-implantitis lesions different from periodontitis lesions? Journal of Clinical Periodontology 38: 188-202.
- Schwarz F, Sager M, Becker J (2011) Peri-implantitis defect model. In: Giannobile WV and Nevins M (eds.). Osteology guidelines for oral and maxillofacial regeneration. 1st edn. Quintessence Publishing, London, UK.p: 197.
- Martini L, Fini M, Giavaresi G, Giardino R (2001) Sheep model in orthopedic research: a literature review.Comparative Medicine 51: 292-299.
- Hickey JS, O'Neal RB, Scheidt MJ, Strong SL, Turgeon D, et al. (1991) Microbiologic characterization of ligature-induced peri-implantitis in the microswine model. Journal of Periodontology62: 548-553.
- Schwarz F, Iglhaut G, Becker J (2012) Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. Journal of Clinical Periodontology 12: 63-72.
- Alhag M, Renvert S, Polyzois I, Claffey N (2008) Re-osseointegration on rough implant surfaces previously coated with bacterial biofilm: an experimental study in the dog. Clinical Oral Implants Research 19: 182-187.
- Kolonidis SG, Renvert S, H?mmerle CH, Lang NP, Harris D, et al. (2003) Osseointegration on implant surfaces previously contaminated with plaque. An experimental study in the dog. Clinical Oral Implants Research 14: 373-380.
- Weinberg MA, Bral M (1999) Laboratory animal models in periodontology. Journal of Clinical Periodontology 26: 335-340.
- Draper HH (1994) Bone loss in animals. Advances in Food and Nutrition Research 9: 53-71.
- Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG (2007) Animal models for implant biomaterial research in bone: a review. Journal of European Cells and Materials 13:1-10.
- Mardas N, Dereka X, Donos N, Dard M (2014) Experimental model for bone regeneration in oral and cranio-maxillo-facial surgery. Journal of Investigative Surgery 27: 32-49.
- Wang S, Liu Y, Fang D, Shi S (2007) The miniature pig: a useful large animal model for dental and orofacial research. Oral Diseases 13: 530-537.