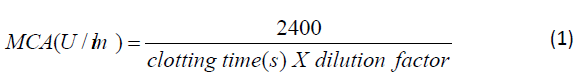Keywords
Solanum dubium seeds; Milk clotting activity; Extraction; Enzyme;
Chromatography; Chymotrypsin; Serine protease
Introduction
Milk production in Sudan is economically important due to its
contribution to human nutrition. The Ministry of Animal Wealth
estimates the annual production of 5.1 million tons of milk as
appears in the FAO report [1]. Cheese-making has survived, as an
art, for more than 7000 years [2]. The advancement of scientific
knowledge has led to a better understanding of the raw material
milk and the cheese-making and ripening process. In Sudan
“given bayda” is unique among cheese varieties as having a high
concentration of table salt added to milk before processing [3].
Since cheese is manufactured from raw or heated milk [4]; it
becomes important to subject it to the salting process to avoid
microbial contamination especially under tropical conditions
[5]. The worldwide increase in cheese production, alongside the
reduced supply of calf rennet, had promoted research towards a
search for alternative sources of milk coagulants [6,7]. Plant or
microbial rennet produced by genetically engineered bacteria and molds provided substitutes for rennin [8]. Early, much research
interests have been directed towards discovering a milk-clotting
enzyme from natural plants [9,10] that could satisfactorily replace
commercial rennet in cheese making [11]. Rennet substitutes
of plant origin have been increasingly used to manufacture
cheese, especially at the putative artisanal level. Application
of plant coagulants allows targetted cheese production and
hence contributes to improving the nutritional input of those
populations on whom restrictions are imposable by the use of
animal rennets [12]. Aqueous fruit extracts from S. dubium were
used traditionally as coagulants in the making of white and soft
cheese in Sudan [13]. Other species of the Solanum genus such as S. incanum. L, S. esculentum, S. macrocarpon. L and S. melongena. L have shown the ability to clot milk [14,15]. Solanum dubium
Fresen is a well-known species belonging to the Solanaceae
family. Therefore, this study focused on the establishment of the
extracted purification enzyme from S. dubium plant seeds which
play a role in milk coagulation activity.
Materials and Methods
S. dubium seeds, used in this investigation, were collected from
west of Omdurman city, Khartoum state, Sudan. Two types of
seeds were collected; one in a full ripe stage (yellow seeds) and
the other in an unripe condition (green seeds). The seeds were
cleaned, dried under room condition, and kept in paper bags until
use.
Sample preparation of extraction
The clotting activity was measured according to the method
described by Arima and Iwassaki [16] with slight modification
which excluded the addition of calcium. A small amount of the
crude extract (50-100 ml) was taken the extracts and mixed with
2 ml of 50% milk (fresh milk diluted in sterile distilled water pH
5.4) at 55oC until clotting was achieved. Milk-clotting activity
(MCA) was calculated according to equation (1).
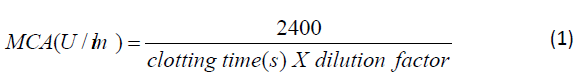
where U is the unit activity which is defined as the quantity of
crude protein in ml needed to coagulate 2 ml of 50% milk at 55°C
within 40 min.
Partial purification of rennin-like enzyme:
Ammonium sulphate precipitation of rennin-like
enzyme
The seed extract of S. dubium was further subjected to
separate steps of precipitation using 0-30%, 30-50%, and 50-
80% concentration by the gradual addition of solid ammonium
sulfate and was allowed to stand submerged in ice for 30 min.
It was then centrifuged at 13,000 rpm for 20 minutes at 4oC.
The obtained protein pellets were dissolved in 50 mM sodium
phosphate buffer (pH 7.1) and the protein obtained was collected
and combined as one fraction. Ammonium sulphate (30% - 55%)
precipitation was repeated and the protein produced was pooled
again in one fraction and dialyzed for 24 hours at 4oC and then
follow by desalting, The membranes containing the 30% - 55%
concentration precipitate were immersed in a beaker containing
sodium phosphate buffer pH (7.1) for 8 hours with continuous
stirring and occasional change of the buffer. The precipitated
enzyme samples were subjected to Sephadex G-75, anion
exchange, and superdex G-75 column chromatography.
Purification of the enzyme using
chromatography column
The protein samples obtained after Sephadex G-75 were subjected
to anion exchange chromatography using HI Trap 2 cm × 5 cm
column (Bio-Rad Laboratories Inc.), previously equilibrated with
a mixture of 50 mM sodium phosphate and 1 mM EDTA (pH 7.1).
The protein fractions were loaded and subsequently eluted using
a sodium chloride gradient (1M) in the same buffer with a flow
rate of 1.3 ml/min. 1 ml fractions were collected and monitored
at a wavelength of 280nm. The peaks were collected and small
aliquots (100 μl) were evaluated for milk-clotting activity. Active
peak fractions were pooled and lyophilized and were used in the next step of purification in superdex G-75. Protein samples
(300 μl) obtained, after anion exchange chromatography, was
subjected to gel exclusion chromatography. The column (1.8
cm+25 cm) was packed with superdx G-75, equilibrated with a
mixture of 20 mM sodium phosphate buffer and 0.15 M sodium
chloride (pH 7.1). Elution was performed at a flow rate of 0.2 ml/
min and 1ml fractions were collected. The absorption effect of
the eluent was monitored at a wave-length of 280 nm.
Polyacrylamide Gel electrophoresis
Polyacrylamide gel electrophoresis (PAGE) of the enzymes
was performed by the method of Lammli [17], using 15%
polyacrylamide gel, sodium dodecyl sulfate (SDS), and
β-mercaptoethanol (SDS-PAGA). The protein bands in the gels
were stained with Coomassie Brilliant Blue. The relative molecular
mass of the protein was estimated by a standard protein marker.
Protease activity
Protease activity was assessed using the spectrophotometric
method reported by Sarath et al. [18]. The enzyme solution (100
ml) was added to 100 ml of 1% casein in 50 mM sodium phosphate
buffer (pH 7.1), followed by gentle mixing and incubation at
37oC for 30 min. The reaction was stopped by adding 1.5ml of
TCA, and the mixture was incubated at room temperature for 30
min and was then centrifuged at 13,000 rpm for 5 min at 4oC. A
control was prepared in the same way, but with the addition of
TCA to stop the enzyme activity. The absorption of the enzyme
was examined at a wavelength of 280 nm. One unit of the
enzyme activity was defined as the amount of the enzyme which
increases the absorption by 0.001 nm at a wavelength of 280 nm
under assay conditions.
Protease activity by the agar plate
The agar plate method [19] may be used for the determination
of the protease activity. The Agar 15g was dissolved in 150 ml
of a mixture which consisted of 20 mM Tris-HCl buffer (pH 8.5),
2 mM calcium chloride and 0.5% casein solution. The mixture
was autoclaved, cooled down to a reasonable temperature,
distributed into 25ml Petri dishes, and was allowed to cool down
and solidify. Wells of 0.8cm diameter were made in the solidified
agar. Each well in a separate agar plate was treated by the addition
of 100ml of either ammonium sulfate precipitate or Superdex
G-75 purified enzyme; a standard was made which contained a
micro quantity of chymosin. The plates were incubated at 37°C
for 20 hours.
Protease activity detection zymography
Enzyme activity was detected by the zymography technique
described by Vladimir et al. [20]. In this technique a purified
enzyme (20 ml) was added to 1 ml of 0.125 M Tris HCl buffer (pH
8.0) containing 5% (w/v) SDS, 1% (w/v) sucrose, and 0.05% (w/v)
bromophenol blue. Electrophoresis (SDS-PAGE) was conducted
in a resolving gel and a stacking gel having all specifications
described earlier in section 2.8.2 and 2.8.3; in addition to 1%
casein. The gel was washed twice with 2% (v/v) Triton X-100 for 30
min. The gel was then transferred into 0.05 M Tris–HCl buffer, (pH 7.5) containing 15 mM CaCl2. The enzyme activity was revealed as
translucent bands in the gel. The band was further visualized by
subjecting the gel to staining and destaining procedure.
Effect of temperature on the enzyme activity
The optimum temperature of protease activity was determined
by conducting the assay at different temperatures in 50 mM
sodium phosphate buffer (pH 7.1). The reaction mixture was
incubated for 30 minutes at various ranges of temperatures from
20°C to 90°C, taking readings every 5°C. The temperature of the
incubation mixture was maintained at 60°C and was left for 1, 2,
3, and 4 hours before the activity was re-measured as a test for
temperature effect on enzyme stability.
Effect of pH on enzymes activity
The hydrogen ion concentration (pH) for protease activity was
determined by conducting the enzyme assay at various pH
values. The reaction mixture of the enzyme was incubated for 30
minutes at 37oC in a water bath. Different buffers, such as 50
mM sodium acetate (pH 4.5 to 5.5), 50 mM sodium phosphate
(pH 6-7) and 50 mM Tris-HCl (pH 7.5-9) was used separately [21].
Effects of different concentrations of protease
inhibitors and metal ions on enzyme activity
The effects of various protease inhibitors such as iodoacetate,
phenylmethylsulfonyl fluoride, chymostatin, cocktail
(AEBSF, E6, pepstatin A, 1, 10-phenanthroline, bestatin, and
ethylenediaminetetraacetic acid (EDTA) on the hydrolyzing ability
of purified protease were tested. About 10 μl of the purified
enzyme was pre-incubated at 37°C for 30 minutes, separately, with
each one of the inhibitors (at different concentrations), followed
by the addition of 1% casein and 50 mM sodium phosphate
buffer (pH 7.1). The residual protease activity was then measured
as was explained before (2.9.3). The following salts were used to
determine the influence of metal ions: (ZnCO4), (MgCl2), (MgSO4)
(CuSO4), (LiCl), (KCl), (KF), (NaCl), (ZnCl), (HgCl2), (FeSO4), (COSO4),
(AlSO4) and (CaCl2). The residual protease activity was measured
at a wavelength of 280 nm. The activity without the inhibitors
and metal ions was considered as 100%.
Measurement of molecular masses of the
purified enzyme
The molecular mass of the purified enzyme was determined
by the SDS PAGE electrophoresis method. The purified enzyme
(20 ml) and standard proteins with different molecular weights,
(10 KD, 15 KD, 20 KD, 25 KD, 50 KD, 75 KD, 100 KD, 150 KD, and
250 KD) were loaded onto SDS-PAGE. The molecular mass of the
purified enzyme was estimated from the plot of standard value
versus the relative front.
Kinetic activity studies
Michaelis–Menten equation was calculated by plotting the
absorbency readings obtained for the effect of different enzyme
concentrations on casein. The 1/V and 1/S values of the enzymatic
samples were obtained using the Line- Weaver-Burk or double
reciprocal equation [22].
Results and Discussion
The result showed that extraction media used in this study,
whether distilled water or different buffers, clearly indicated
that the latter gave more extractable proteins and consequently
enzymes than the former; with the highest values reached when
sodium phosphate buffer was used. The results also indicated,
that more solid support for the use of the phosphate buffer may
be judged by the total amounts of extractable amino-acid as
indicated by the amino-acid profiles and their comparison with
those obtained by extraction with other media; greater amounts
were obtained in case of the phosphate buffer. Accordingly, the
phosphate buffer was chosen for protein extraction (both crude
and purified extracts) from the seed of S. dubium.
The next step, after establishing the phosphate buffer as a
medium for the extraction of the best obtainable protein, was
to precipitate, purify and characterize the isolated protein. It was
found that the best protein precipitation was achieved by the
use of 30-50% ammonium sulfate concentration for seed; which
makes it within the range of 40-60% concentration for maximum
ammonium sulfate precipitation reported by Lakshmi et al.
[23] using Bacillus subtilis and at the same time closer to those
reported by El-Hofi et al. [24] who applied 40-50% ammonium
sulfate concentration for the precipitation of Carica papaya
protein.
Protein dialysis is another method for increasing the precipitation
fold as was reported by Sarah et al. [25,26] and which was applied
to attain the results presented in this work leading to a decrease
in recovery of proteins, increase in a specific activity, and
purification folds characteristic of a pure and active enzyme as
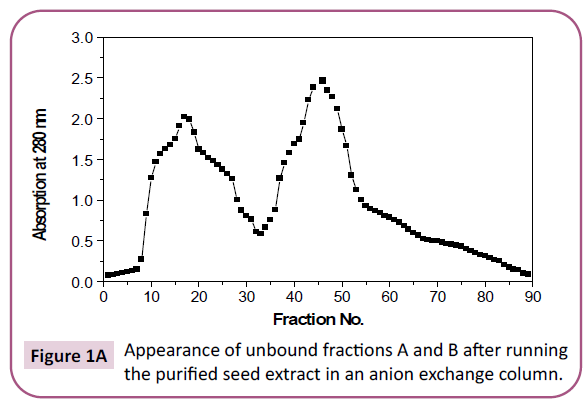
shown in Table 1. Purification of the enzyme by the use of anion
exchange chromatography, which ended up in the separation
of the enzyme into two peaks (Figure 1B), indicated that the
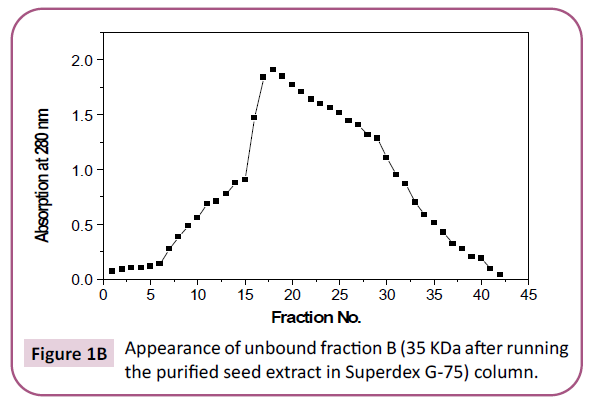
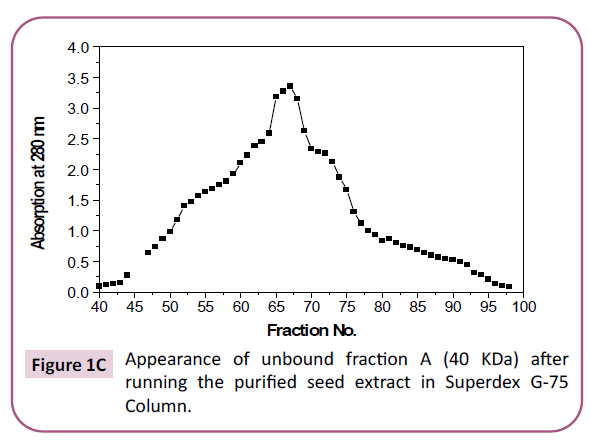
enzyme was still not a homogeneous one. Homogeneity (pure
enzyme) is reached after the enzyme was run through superdex
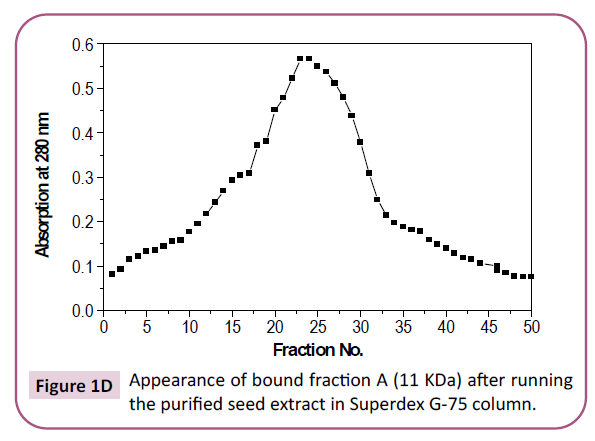
G-75 column where only one peak was detected (Figure 1C). On
the other hand, heterogeneity with the appearance of more than
one peak was noticed for the enzymes separated by the sephadex
(Figure 1A) and ion exchange columns; which, ultimately suggests
their impurity. Support for the separation by the pure enzyme
with a single peak may be drawn from the work carried out on
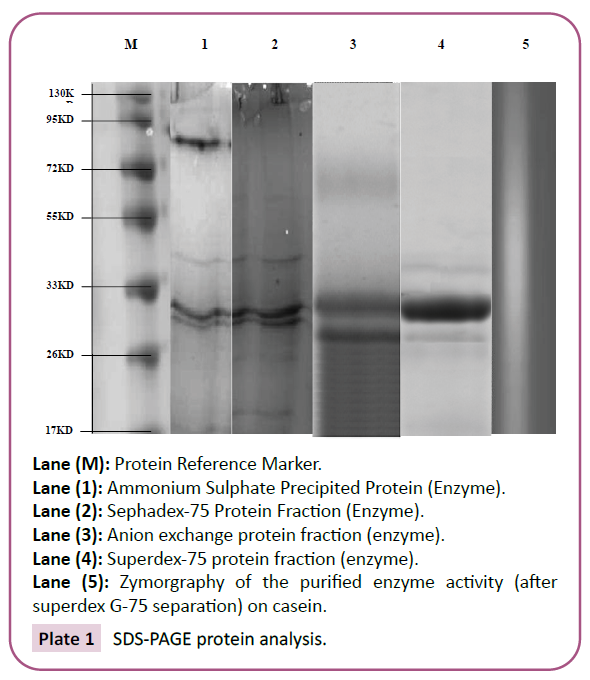
SDS-PAGA (Plate 1). The single homogenous enzyme produced by
superdex-G-75 proved to be highly active and leads to complete
hydrolysis of casein.
Purification
Step |
Total Protein
(mg/ml) |
Total Activity
(units) |
Specific Activity
(units/mg) |
Recovery
(%) |
Purification (fold) |
| Crude Extract |
003.2 |
68.571 |
21.428 |
100 |
1.00 |
| NH4SO4 Precipitation |
0-30% |
200.0 |
8.888 |
44.4 |
13 |
2.07 |
| 30-50% |
962.0 |
57.831 |
60.1 |
84 |
02.80 |
| 50-80% |
271.0 |
10.00 |
36.9 |
15 |
01.7 |
Second
NH4SO4 |
30-55% |
512.0 |
61.40 |
119.9 |
89 |
05.5 |
| Dialysis |
477.4 |
52.00 |
108.0 |
76 |
05.0 |
| Sephadex G-75 Run |
390.0 |
45.00 |
115.0 |
65 |
05.3 |
| Anion Exchange Peaks |
Unbound A |
300.0 |
43.00 |
143.0 |
62.0 |
06.7 |
| Unbound B |
277.0 |
40.00 |
144.0 |
58.0 |
06.7 |
| Bound C |
215.0 |
38.00 |
176.0 |
56.0 |
08.00 |
| Superdex G-75 Peaks |
Unbound A |
130.0 |
34.280 |
261.53 |
49 |
12.1 |
| Unbound B |
132.0 |
26.666 |
262.20 |
38 |
09.4 |
| Bound C |
115.0 |
25.945 |
225.60 |
37 |
10.5 |
Table 1 Total proteins, total activity, specific activity, recovery, and purification fold of the enzyme at different concentrations and purification steps.
Figure 1a: Appearance of unbound fractions A and B after running the purified seed extract in an anion exchange column.
Figure 1b: Appearance of unbound fraction B (35 KDa after running the purified seed extract in Superdex G-75) column.
Figure 1c: Appearance of unbound fraction A (40 KDa) after running the purified seed extract in Superdex G-75 Column.
Figure 1d: Appearance of bound fraction A (11 KDa) after running the purified seed extract in Superdex G-75 column.
Lane (M): Protein Reference Marker.
Lane (1): Ammonium Sulphate Precipited Protein (Enzyme).
Lane (2): Sephadex-75 Protein Fraction (Enzyme).
Lane (3): Anion exchange protein fraction (enzyme).
Lane (4): Superdex-75 protein fraction (enzyme).
Lane (5): Zymorgraphy of the purified enzyme activity (after
superdex G-75 separation) on casein.
Plate 1: SDS-PAGE protein analysis.
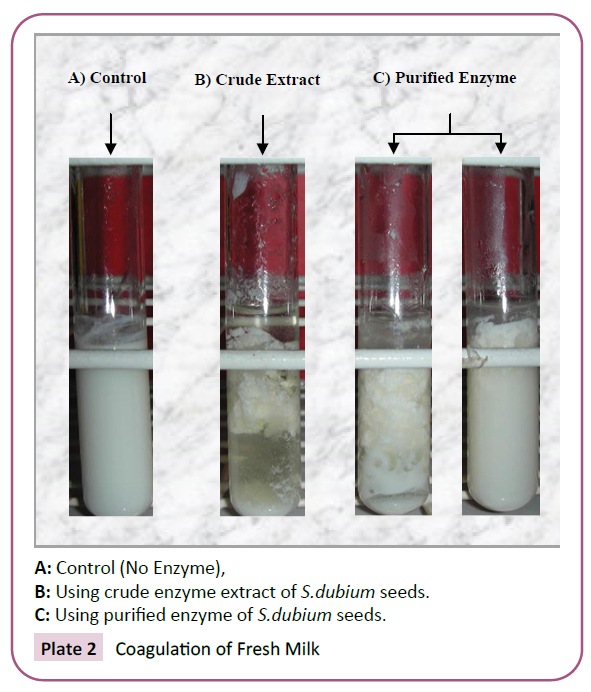
The primary stages of milk coagulation mainly involve the
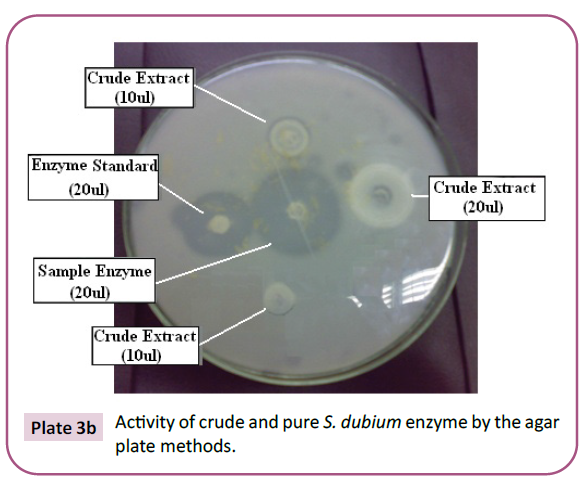
hydrolysis of casein (Plate 2 and Plate 3). It is, therefore, important
to estimate, quantitatively, how the rate and the coagulant activity
are affected by some determining factors. Pure, rather than
crude enzyme extracts were subjected to clotting ability test in
order to exclude the presence of any possible inhibitors. The time
needed for milk clotting pure enzyme seed counterpart reported
in this study might be ex-plained by the possibility that some
of the extracted enzymes, may still be in the pro-enzyme state
as in the case of the soaked seeds which, is a well-established
phenomenon. This assumption is consistent with what happens to the enzyme amylase in dormant starchy seeds where only
β-amylase shows immediate activity, while α-amylase becomes
active as the seed germinates [27].
A: Control (No Enzyme),
B: Using crude enzyme extract of S.dubium seeds.
C: Using purified enzyme of S.dubium seeds.
Plate 2: Coagulation of Fresh Milk
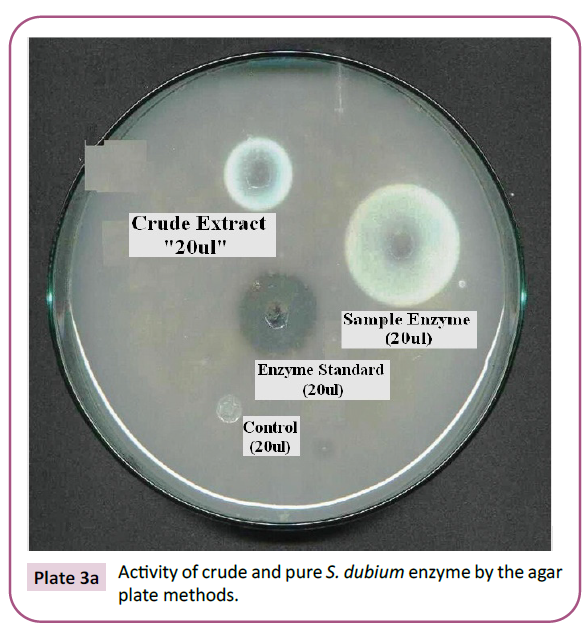
Plate 3a: Activity of crude and pure S. dubium enzyme by the agar plate methods.
Plate 3b: Activity of crude and pure S. dubium enzyme by the agar plate methods.
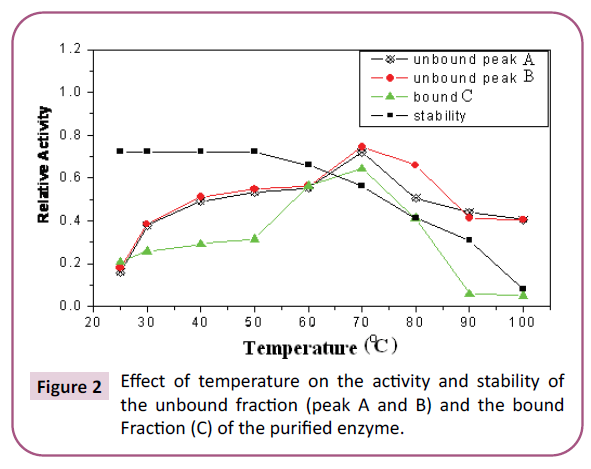
Temperature plays a central role in the protease or clotting
enzyme activity and a wide range of temperatures was suggested
as an optimum for high enzyme activity (Figure 2). The optimum temperature reported was about 60°C for the enzyme extracted
from B. Hispidia var Ryukya [28] and about 55°C for Crustacean
Muruid enzyme by Ambrosio et al. The results obtained in this
work are similar to those obtained by Mohamed-Ahmed et al.
[29] with an optimum temperature upto 70°C which is not far
from some other protease of Aspergillus niger with an optimum enzyme because most enzymes are unstable at alkaline pH. The
use of certain inhibitors (Table 2) may provide an insight into the
nature of the enzyme, its cofactors and the nature of the active
center [30-32]. The description of the purified enzyme from S.
dubium seeds and callus tissues as a serine protease is supported by its inhibition by PMFS [33] and that it reacted to the inhibitor
in a way similar to the reaction of chymstain, which is known
as a serine protease. On the other hand, iodoacetate and the
cocktail mixture (AEBSF, E-6, pepstatin A, 1, 10-phenan throline
and bestatin) did not inhibit the enzyme, thus providing a further
proof for its serine nature, since the use of these inhibitors affect
non-serine proteases [34].
Figure 2: Effect of temperature on the activity and stability of the unbound fraction (peak A and B) and the bound Fraction (C) of the purified enzyme.
| Inhibitors |
Concentration(mM) |
Relative Activity of Enzymes |
| Control |
None |
100 |
| Iodoacetate (1mM) |
1.0 |
83 |
| 2.0 |
90 |
| PMSF |
1.0 |
35 |
| 2.0 |
27 |
| Sigma Cocktail |
1.0 |
102 |
| 2.0 |
112 |
| Chymostain |
0.001 |
51 |
| EDTA |
1.0 |
96 |
| 2.0 |
100 |
Table 2 Effect of different inhibitors on the purified enzyme activity.
The effects of different metal ions (Table 3) on the enzyme activity
from seeds gave results that may be divided into three groups.
The first group is for the CaCl2, effect which increased the activity
and is in agreement with the work of Whitaker on Finin, Sheded
on Solanum terbium and Osman on S. dubium [35-37]. The
effect is attributed to the Ca2+ ions which are known to enhance
protease activity and stability at various concentrations [38]. The
second group (CuSO4, HgCl2, ZnSO4, Zn Cl2, and COSO4) exhibited
a decrease in enzyme activity; a result which is supported by the
work of Habbani [39]. Also, inhibition or reduction of activity
was reported by Lynn and Radford [40], for Hg2+, Zn2+, Mg2+, Mn+,
and Ca2+ ions. The third group is not very much affected by the
addition of the metal (Na1+ and K1+) ions and gave results that
are more or less similar to the control in case of the seed extract,
and consequently in agreement with the results of Mohamed-
Ahmed et al. [29]. On the contrary, the callus tissue enzyme
reacted differently to the presence of Na1+ ion with increased
activity of the enzyme.
| Metal Salts |
Residual Activity % |
Metal Salts |
Residual Activity % |
| Control |
100 |
KF |
104 |
| KCl2 |
102 |
MgSO4 |
098 |
| CuSO4 |
050 |
HgCl2 |
050 |
| Li Cl |
109 |
FeSo4 |
080 |
| Na Cl |
103 |
CoSO4 |
060 |
| CaCl2 |
121 |
MgSO4 |
101 |
| ZnCl2 |
053 |
ZnSO4 |
052 |
| MgCl2 |
099 |
AlSO4 |
097 |
Table 3 Metal salts compound at 100 mM concentration on the enzyme
activity.
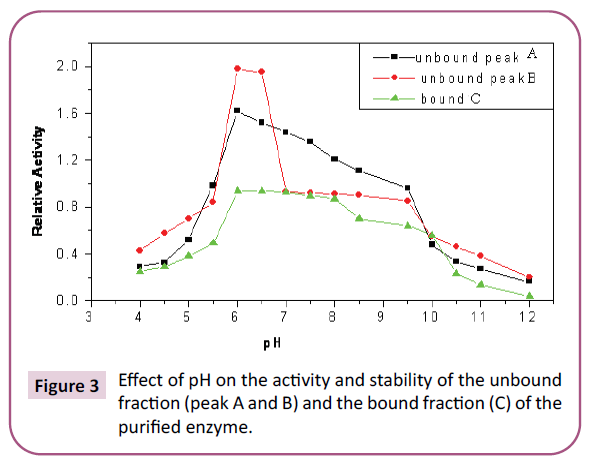
As for the effect of pH on the enzyme activity (Figure 3), the
results described as “optimum” (6-8) for extracted unbound
apure enzyme are close to those obtained by Ambroso et al., who
reported an optimum pH between 6.5 and 8.5. It also coincides
with the pH optimum observed for the bound enzyme extracted
from callus tissue (7.5-9.5). However, the results presented
in this study are lower than those obtained by Yoon et al. [31]
who reported a pH value of 7.5-9 concentration. The substantial
difference in pH range (6-10) for extracted purified bound may
be attributed to the difference of the enzyme precursor, plant
part, and the extraction and purification methods. The results
conclude that the isolated enzyme from S. dubium is a unique temperature reaching up to 60°C [30]. The stability of the enzyme
started to decrease when the temperature approached 70°C
after four hours of incubation which is in agreement with work
reported by Mohamed-Ahmed et al. [29]. These results indicated
that the obtained proteases act at a broad temperature range
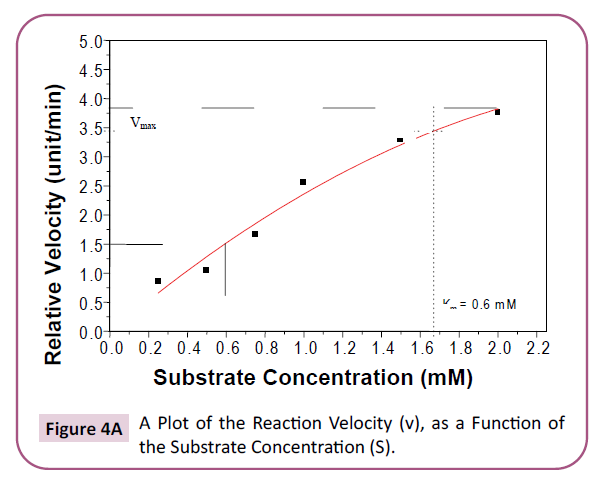
(50°C -70°C), which offers potential application in many fields. The enzyme affinity to the substrate is measured by the Km
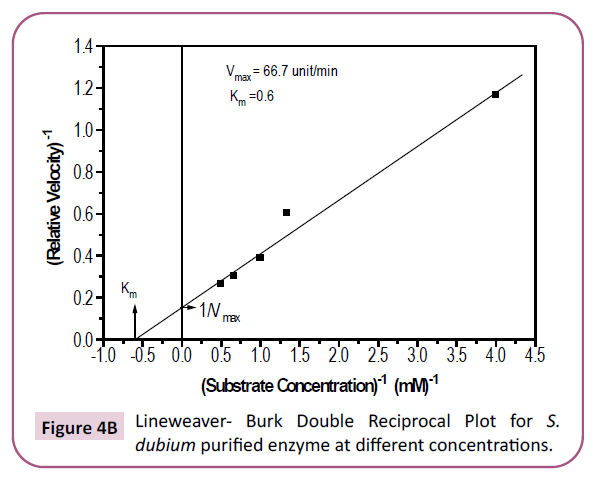
value, bearing in mind the fact that smaller values indicate
greater affinity [41]. It is clear, from the results obtained in this
work, that the Km values are almost 1/3 of those obtained by
Hidayatalla [42] for Caesalpinia protease enzyme and hence of
greater affinity for the substrate than the latter. The Km values
are also lower than those obtained for some other enzymes like
field bean seeds enzyme (10.5) and spinach (3.13) reported by
Paul et al. [43] and Golbeck et al. [44] respectively (Table 4).
Figure 3: Effect of pH on the activity and stability of the unbound fraction (peak A and B) and the bound fraction (C) of the purified enzyme.
| Substrate |
Concentration
(%) |
Monitored at Wavelength 280nm |
Relative Activity |
| Casein |
1.0 |
280 |
100 |
| BSA |
1.0 |
280 |
30 |
| Gelatin |
1. |
280 |
50 |
| Azocasein |
1.0 |
280 |
62 |
Table 4 Substrate, concentration on the specificity of the pure S.dubium
enzyme.
The Km represents the maximum rate attained when the enzyme
sites are saturated by the substrate of the enzyme Therefore,
the enzymes are similar from the enzyme Kinetic point of view.
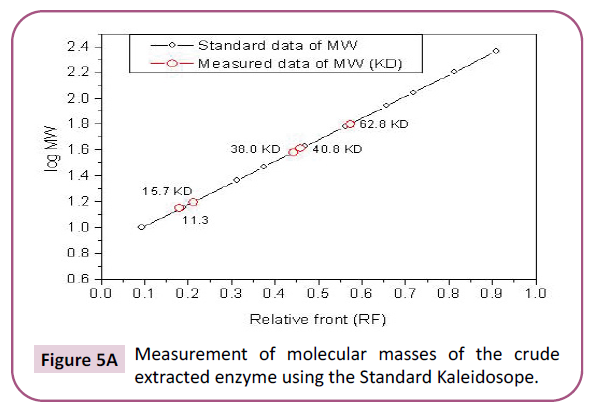
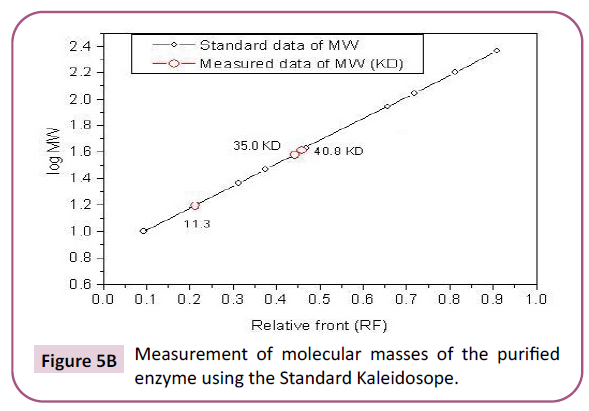
Estimation of crude extract molecular masses revealed that an
enzyme from S. dubium seeds has five unite of molecular masses
(11.3 KDa, 15.7 KDa, 38 KDa, 40 KDa, and 62.8 KDa), while those
of the purified enzyme has three only (11.3 KDa, 35 KDa, and
40 KDa) (Figures 4A and 4B). These results are similar to those obtained by Lynn and Radford [40] who extracted a serine
protease from the latex of Hevea brasiliensis. They are also close
to those obtained for different parts of S. dubium subjected to
SDS-electrophoresis with molecular masses within the range of
27-45 KDa [37]. However, other molecular masses reports are
available for a well-known plant serine protease, from the Cucumisin
family, in the range of 60-70 KDa (Figures 5A and 5B) [45-47].
Figure 4a: A Plot of the Reaction Velocity (v), as a Function of the Substrate Concentration (S).
Figure 4b: Lineweaver- Burk Double Reciprocal Plot for S. dubium purified enzyme at different concentrations.
Figure 5a: Measurement of molecular masses of the crude extracted enzyme using the Standard Kaleidosope.
Figure 5b: Measurement of molecular masses of the purified enzyme using the Standard Kaleidosope.
Results obtained from Blast search are highly indicative of the
identification of the purified protein as a serine protease. This
results is comparable to results recently reported by Mohamed-
Ahmed et al. [29]. The authors reported a 14 residue sequence
of a dubiumin; a chymotrypsin-like serine protease from seeds
of S. dubium freshens. The first ten residues of the reported
sequence demonstrate a 100% identity to results obtained in
this investigation and demonstrate 100% identity to the first
ten residues of Lycopersicon esculentum and slightly lower
magnitudes of identity to other plant serine proteases, for
example, 90% for Alnus species [47] and 80% for Lily and Canein
species [48].
Conclusion
Purified enzymes play a highly significant role in the milk-clotting ability and they are agents for use in potential food and bioindustrial
raw material. The presented in this study may be
concluded as follows: The best buffer medium for extraction is
sodium phosphate buffer at a pH 7.1. It was found that CaCl2 enhances the activity of the enzyme; which explains why CaCl2 is used in the cheese industry. The enzyme is thermostable up
to 70°C and works well within a pH range of 6-7 concentrations.
The purified enzyme was recorded by molecular mass analysis
indicates that the enzyme is similar to chymotrypsin-like serine
protease.
Acknowledgements
I acknowledge to UNESCO_L’OREAL Co-Sponsored Fellowships
programs for Young women and acknowledge the National
University of Malaysia (UKM) for the great support
References
- Mahgoub GZ (2000) Country Pasture/Forage Resource Profiles. Fao Report. http://www.fao.org/ag/Sudan.
- Oۥ connor CB (1993) Traditional cheese making manual ILCA, International livestock centre for Africa, Addis Ababa. Ethiopia.
- Osman AO, Owni EL, Omer AI, Hamed (2007) Production of White Cheese (Gibna bayda) in Zalingei Area West Darfur (Sudan). Aurstialna Journal of Basic and applied science 1: 756-762.
- Ibrahim A (2003) Effect of processing and storage condition on the chemical composition and microbial quality of white soft cheese. M.Sc. Thesis University of Khartoum, Sudan.
- AllaGabo HI (1986) Studies on composition and quality of Gibna Beyda. M.Sc. Thesis, University of Khartoum, Sudan.
- Cavalcanti MT, Teixeira MF, Lima-Filho JL, Porto AL (2004) Partial purification of New milk clotting enzyme produced by Nocardiopsis sp. Journal of Bioresource Technology 93: 29-35.
- Shah MA, Mir SA, Paray MA (2014) Plant proteases as milk-clotting enzymes in cheesemaking: A review. Dairy Science and Technology 94: 5-16.
- Eco M (2008) Kill a calf and make a cheese. Available online since August 2008 at website: http://www.care2.com/rewards/.
- Egito AS, Girardet JM, Laguna LE, Poirson C, et al. (2007) Milk-clottingactivity of enzyme extracts from sunflower and albizia seeds and specific hydrolysis of bovine κ-casein. International Dairy Journal 17: 816-825.
- Guiama VD, Libouga DG, Ngah E, Mbofung CM (2010) Milk-clotting activity of berries extracts from nine solanum plants. African Journal of biotechnology 9: 3911-3918.
- Ibrahim AE, Mostafa N, Ahmed AM, Amira R, et al. (2009) Alkaloid Production and Organogenesis from Callus of Hyoscyamus muticus L. In vitro. Journal of Applied Sciences Research 5: 82-92.
- Silva SV, Malcata FX (2005) Partial identification of water soluble peptides released at early stages of proteolysis in sterilized ovine cheese-like systems: Influence of type of coagulant and starter. J Dairy Sci 88: 1947-1954.
- Yousif BH, McMahon DJ, Shammet KM (1996) Milk-clotting Enzyme from Solanum dobium Plant. Int. Dairy J 6: 637-644.
- Guiama VD, Libouga DG, Ngah E, Beka RG (2010b) Milk-clotting potential of fruit extracts from Solanum esculentum, Solanum macrocarpon L. and Solanum melongen. African Journal of Biotechnology 9: 1797-1802.
- Sulieman YR, El-Imam YM, Allagabo HI (1988) Milk coagulating properties of Solanum Incanum. Sudan Journal of Anim Prod 1: 109-112.
- Arima K, Iwasaki S, Pearlman EG, Lorand L (1970) Milk clotting enzyme from Mucor Pusillu (Eds.) Methods in enzymology, Academic Press, New York, USA 446-459.
- Laemmli UK (1970) Cleavage of Structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- Sarath G, Delamotte R, Wagner F (1989) Prolease assay methods. In proteolytic enzyme. A practical approach (Beynon, R. and Bond, J. Eiditors) Pubblished by IRL Press, Oxford 25-56.
- Rebecka M, Alexander B, Sonja K, Anitha L (2019) Agar plate‐based screening methods for the identification of polyester hydrolysis by Pseudomonas species, Microbial Biotechnology13: 274-284.
- Vladimir RK, Kenneth JM (2003) Expanding the Use of Zymography by the Chemical Linkage of Small, Defined Substrates to the Gel Matrix. Genome Research 1961-1965.
- Esteves CL, Lucey JA, Wang T, Pires EM (2003) Effect of pH on gelation properties of skim milk gels made from plant coagulants and chymosin. J Dairy Sci 86: 2558-2567.
- Jatati RD, Pranab KD, Rintu B (2005) Kinetic study of a low molecular weight protease from newly isolated pseudomonas sp. Using artificial neural network. Indian Journal of Biotechnoology 127-133.
- Lakshmi G, Prasad NN (2015) Purification and characterization of alkaline protease from a mutant Bacillus licheniformis Bl8. Adv Biol Res 9: 15–23.
- El-Hofi MA, Ismail AA (2000) Utilization of purified and characterized lipase from papaya Carica papaya in Acceleration of Rass cheese slurry. Egypt J Food Sci: 61-72.
- Mahjoub S (2002) Studies on the physiological, environmental and biochemical factors affecting the germination of seed of forest tree species. PhD Thesis, Department of Botany,University of Khartoum, Khartoum, Sudan.
- Sarah MA, Julie AT, Louis Z (2002) Dialysis and concentration of protein solutions. Curr Protoc Toxicol 3: 1-5.
- Bialecka B, Kepczynski J (2010) Germination α,β-amylase and total dehydrogenase activity of Amaranthus Caudatus seeds under water stress in the presence of Ethephon and Gibberellin A3. ACTA Biologica Carcoviensia Series Botanica 52: 7–12.
- Uchikoba T, Yonezawa H, Kaneda M (1998) Cucumisin- like protease from sarcocarp of Benincase hispide var. Ryukyu. Phytochemistry 49: 2215-2219.
- Ahmed IA, Morishima I, Babiker EE, Mori N (2009) dubiumin, a chymotrypsin-like serine protease from the seeds of Solanum dubium resen. Phytochemistry 70: 483–491.
- Rayda S, Alya SK, Mohamed H, Ines A (2009) Extracellular acid protease from Aspergillus niger I1: purification and characterization. African Journal of Biotechnology 8: 4582-4589.
- Yoon HL, Soung SK (1985) Purification and properties of glucose dehydrogenase from tobacco callus culture. Korean Biochem J 19: 424-432.
- Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79: 544-552.
- Mohammed AI, Isao M, Elfadil EB, Nobuhiro M (2009) Characterization of partially purified milk-clotting enzyme from Solanum dubium frozen seeds. Food Chem 116: 395-400.
- Kumar A, Sharma J, Kumar A, Sunita MG (2006) Purification and characterization of milk clotting enzyme from goat Capra hircus. India. Journal of comparative Biochemistry and physiology 108-113.
- Whitakar JR (1959) Properties of the milk clotting enzyme of Ficus carica. Food Tech 13: 86-94.
- Sheded AM (1975) Effect of vegetable rennet on some characteristics of cheese. Master Thesis. University of Cairo, Egypt.
- Osman MS (1996) Purification and characterization of milk clotting protease from Solaum dubium. M.Sc. Thesis, University of Khartoum, Sudan.
- Kannan Y, Koga Y, noue Y, Haruki M (2001) On Active subtilisin –like protease from a hyperthermophilic archaeon in a form with aputative prosequence. App Environ Microbiol 67: 2445-2452.
- Habbani ES (1992) A study of plant rennet extracted from Solanum dubium (Gubbain) M.Sc. Thesis, University of Khartoum, Sudan.
- Lynn KR, Radford CN (1984) Purification and characterization of hevain, a serine protease from Hevea brasiliensis Division of Biological Sciences, National Research Council of Canada, Ottawa Canada K1A OR6.
- Siddig M, Sinha NK, cash JN (1992) Characterization of polyphenoloxidase from Stanley plums. Journal of food science 57: 1177-1179.
- Hidayatallah K (2006) Purification and characterization of serine protease from seed Caesalpinia bonducella. PhD Thesis, University of Science and Technology. HEJ Research Institute of Chemistry, University of Karachi, Paskistan.
- Paul B, Gowda LR (2000) Purification and Characterization of a Polyphenol Oxidase from the Seeds of Field Bean (Dolichos lablab). J Agric Food Chem 48: 3839-3846.
- Golbeck JH, Cammarata KV (1981) spinach thylokoid polyphenol oxidase isolation, activation and properties of the native chloroplast Enzyme. Plant Physiol 67: 977-984.
- Bernardo R, Yuridia M, Cesar HR Lourdes VT (2004) Purification and characterization of a serine carboxypeptidase from Kluyveromyces marxianus. Inter J of food Micro 91: 245-252.
- Debora F, Debora F. and Berne J.(2002) SEP-1-A Subtitisin –Like serine endopeptides from germinated seeds of hordeum Vulgate L. cv. Morex. Planta 215:885-895.
- Ribeiro A, Akkermans A, Kamen A, Bisseling T (1995) Anodule- specific gene encoding asubtilisin-like protease is expressed in early stages of actinorhizal nogule development. Plant Cell 7: 785-794.
- Patel AK, Singh VK, Jagannagham MA (2007) Carnein, Aserine protease from noxious plant weed Ipomoea cornea (Morning Glory). Journal of Agricultural and Food Chemistry 55: 5809-5818.