Ravi Ranjan1*, Manjula Karpurapu2, Asha Rani1, Athar H Chishti3 and John W Christman2*
1Department of Medicine, University of Illinois, Chicago, Illinois, USA
2Department of Internal Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep, The Ohio State University, Columbus, Ohio, USA
3Department of Developmental, Molecular and Chemical Biology, Tufts University School of Medicine, Boston, USA
Corresponding Author:
Dr. John W Christman
Department of Internal Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep, The Ohio State University, 201 Davis Heart Institute, Columbus, OH 43210, USA.
Tel: 6142477804
E-mail: John.Christman@osumc.edu
Dr. Ravi Ranjan
Department of Medicine, University of Illinois, Chicago, Illinois, USA.
Tel: 3128049896
E-mail: ranjan@uic.edu
Received date: June 24, 2016; Accepted date: August 09, 2016; Published date: August 14, 2016
Citation: Ranjan R, Karpurapu M, Rani A, et al. Hemozoin Regulates iNOS Expression by Modulating the Transcription Factor NF-κB in Macrophages. Biochem Mol Biol J. 2016, 2:2. doi: 10.21767/2471-8084.100019
Keywords
Hemozoin; Inducible nitric oxide synthase; Lipopolysaccharide; Macrophage; Malaria; Sepsis
Abbreviations
Hz: Hemozoin; NF-κB: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; iNOS: Inducible Nitric Oxide Synthase; IFNγ: Interferon Gamma; LPS: Lipo-Polysaccharide
Introduction
Malaria is one of the most devastating diseases worldwide with high incidence of morbidity and mortality [1]. The co-infection of malaria and Gram-negative bacterial sepsis is also a major concern that results in severe clinical complications [2-4]. Hemozoin (Hz) is released following the rupture of the erythrocytes, which is formed by the parasite – Plasmodium sp., as a detox mechanism during the intra-erythrocytic cycle [5]. Macrophages (in particular liver-resident) play an important role in elimination of malarial parasites and subsequent phagocytosis of hemozoin [6,7]. The release of hemozoin has physiological relevance as it can modulate the immune system, and it may continue to be present in many organs, such as liver, spleen for extended period in the life time [8]. Hemozoin also induces lung inflammation and correlates with acute respiratory distress syndrome, and may impact the pulmonary microvasculature [9,10]. Hemozoin is reported to modulate the immune functions of immune cells such as macrophages and dendritic cells. Some reports suggest Hz as an inert, bystander; other suggests contradictory findings as immune-activating or suppressive effects [11-14]. Macrophages are also one of important components of the immune system being involved in both the innate and adaptive immune responses. Macrophages are the major phagocytotic cells of the immune system and are essential for generating beneficial inflammation that is necessary for host defence and bactericidal activity [15,16]. In response to bacterial infection, the molecular signalling events in macrophages leads to expression of iNOS (inducible nitric oxide synthase) and are important sources of iNOS-derived nitric-oxide (NO), which together with the respiratory burst of reactive oxygen species (ROS), leads to effective bactericidal activity [15,17].
The molecular interactions between Hz, bacterial components, inflammatory mediators and macrophages remains poorly investigated. In this study, we investigated the combinatorial immune-modulatory effects of synthetic Hz and bacterial endotoxins and pro-inflammatory agents. Here we report the effect of phagocytosis of hemozoin on subsequent production of iNOS by macrophages in response to pro-inflammatory agents- IFNγ and LPS.
Material and Methods
Culture of RAW 264.7 macrophage cell line
Murine macrophage/monocyte cell line RAW 264.7, was cultured in 1×DMEM media containing 10% FBS (Hyclone) supplemented with penicillin (100 U/mL), streptomycin (100 μg/ml), and maintained at 37°C in a humidified air containing 5% CO2 [18].
Phagocytosis of hemozoin
RAW 264.7 macrophages were treated with Hz (100 μg/ml) and incubated for 3 h and 18 h. The macrophages were also pretreated with Hz and followed by stimulation with pro- and anti-inflammatory agents - IFNγ (100 ng/ml), LPS (1 μg/ml) and IL4 (20 ng/ml) for 8 h, and observed under microscope at 40× magnification (Nikon) for phagocytosis and morphological attributes.
Expression of iNOS by macrophages
Hemozoin crystals (Catalogue # tlrl-hz, InvivoGen) was suspended in sterile molecular grade water, and sonicated in bath sonicator (Bransonic® Ultrasonic Baths, Thomas Scientific) prior to use. RAW 2764.7 macrophages were stimulated with synthetic hemozoin with various concentration 10, 20, 50, 100, and 250 μg/ml for 16 h. LPS TLR-grade (1 μg/ml) (Alexis) was used as positive control. The macrophages were washed with 1×PBS and lysed with 1×RIPA buffer containing protease inhibitor cocktail (539131, Calbiochem) and 1 mM phenylmethylsulfonyl fluoride (PMSF), and processed for immunoblotting, as described before [18]. Antibodies used were anti-iNOS/NOS Type II (610332, BD Transduction Laboratories) and anti-β-actin (Sigma).
Expression of iNOS by macrophages in response to pro- and anti-inflammatory agents
Macrophages were pretreated with hemozoin and stimulated with - IFNγ [100 ng/ml] (BD Pharmingen), LPS [1 μg/ml] (Alexis Biochemicals) and IL4 [20 ng/ml] (RnD Systems), and processed for Western immunoblotting for iNOS as described previously [19].
Effect of hemozoin on transcriptional activity
RAW 2764.7 macrophages were pre-treated with hemozoin (100 μg/ml for 14h) and stimulated with IFNγ (100 ng/ml) and LPS (1 μg/ml) for 15, 30, 60 min. The cells were washed with 1×PBS, and lysed with 1×RIPA buffer, containing protease and phosphatase inhibitor cocktail (539131 and 524625 respectively, Calbiochem) and 1 mM PMSF. The lysates were immunoblotted for phospho- NF-κB p65 (Ser536) (3031S, Cell Signaling) and NF-κB p-65 (3034, Cell Signaling).
Results and Discussion
Effect of hemozoin on expression of iNOS
Previous studies have used Hemozoin (Hz), isolated from parasitized RBCs or synthesized in the research labs to investigate the role of Hz in immune-modulation. The methods described to isolate natural Hz or synthesize Hz are time consuming, laborious, and there could be chance of contamination of the Hz with other cellular components or chemical components [20]. The concentration of Hz used in these experiments ranged from 10 μg/ml to 400 μg/ml for in-vitro cellular assays, and has been previously described [20]. We came across commercial synthetic Hemozoin crystals (Catalogue # tlrl-hz, InvivoGen) and sought to investigate its effects on expression of iNOS in macrophages, as this source of Hz is readily available. Based on the previous literature [20], we chose to use 10 μg/ml to 250 μg/ml of Hz. Macrophages were stimulated with various concentrations of hemozoin (Hz) for 16 h, and immunoblotted for iNOS. Hemozoin had no effect on expression of iNOS, compared to LPS alone, which was used as positive control (Supplementary Figure 1). Other researchers have used Hz isolated from parasitized RBCs and synthetic Hz and observed differing results [8,14,20,21]. The stimulation with different sources of Hz have reported to induce differential gene expression in macrophages [8,14,20,22]. Since we did not observe iNOS expression in response to hemozoin, we checked if hemozoin was phagocytosed by macrophages. Microscopic imaging suggests that Hz was phagocytosed by macrophages in less than 3 h (Figure 1) and did not result in any notable changes in cell morphology.
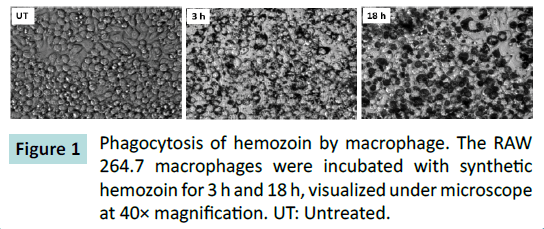
Figure 1: Phagocytosis of hemozoin by macrophage. The RAW 264.7 macrophages were incubated with synthetic hemozoin for 3 h and 18 h, visualized under microscope at 40× magnification. UT: Untreated.
We further investigated the effect of bacterial components (LPS) and inflammatory mediators (IFNγ) as one would expect in a sepsis conditions. The macrophages were, pretreated with Hz and challenged with pro-inflammatory agents (IFNγ or LPS) to mimic the release of inflammatory mediators such as iNOS. An increased expression of iNOS protein was observed when macrophages were pre-treated with Hz, and stimulated with IFNγ when compared to IFNγ alone (Figure 2). Surprisingly, we observed unexpected results. The expression of iNOS was reduced, when macrophages were pretreated with Hz, and then stimulated with LPS, when compared to LPS alone (Figure 2). In controls, the macrophages were pretreated with Hz stimulated with IL4 or IL4 alone (an anti-inflammatory agent), and as expected in both the approach, there was no production of iNOS.
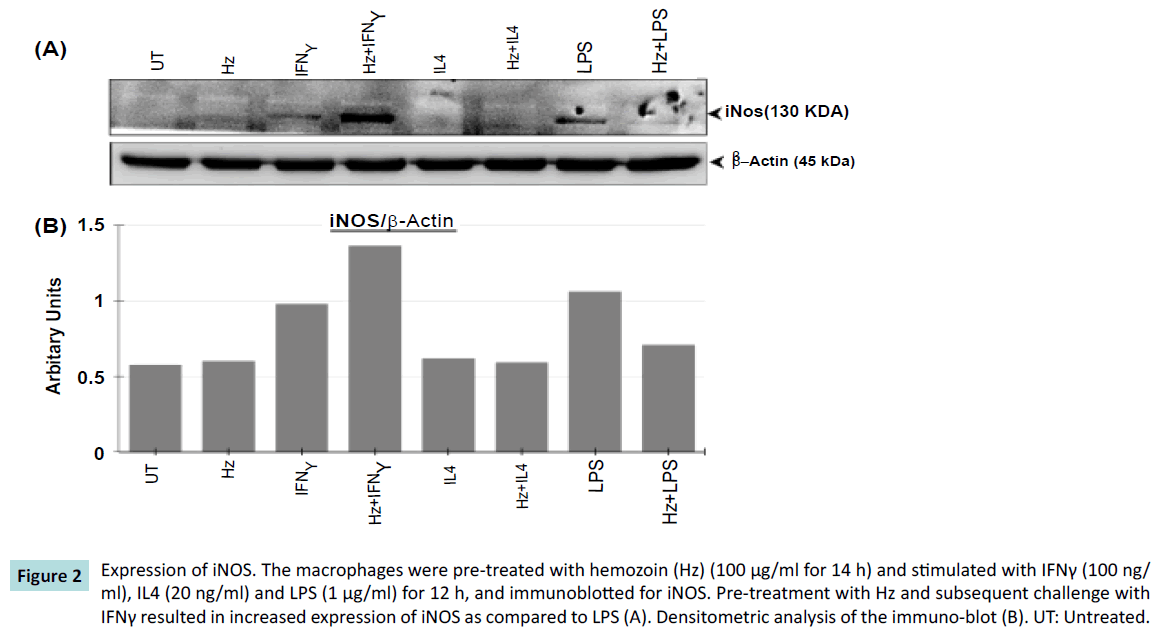
Figure 2: Expression of iNOS. The macrophages were pre-treated with hemozoin (Hz) (100 μg/ml for 14 h) and stimulated with IFNγ (100 ng/ ml), IL4 (20 ng/ml) and LPS (1 μg/ml) for 12 h, and immunoblotted for iNOS. Pre-treatment with Hz and subsequent challenge with IFNγ resulted in increased expression of iNOS as compared to LPS (A). Densitometric analysis of the immuno-blot (B). UT: Untreated.
This result shows that the phagocytosis of the Hz by macrophages alters the immune-modulatory effects, which affects the iNOS production. A similar observation, indicating enhanced iNOS expression when macrophages (RAW 264.7 cells) were fed with Hz and treated with IFNγ has been reported [14]. We further checked, if the pretreatment with Hz and subsequent challenge with IFNγ or LPS or IL4 had any morphological effects on macrophages. There was no apparent change in the morphological appearance of macrophages post Hz phagocytosis (Figure 3).
Figure 3: Effect of hemozoin and pro- and anti-inflammatory agents. Macrophages were treated with hemozoin (100 μg/ml for 14 h) and were stimulated with IFNγ (100 ng/ml), LPS (1 μg/ml) and IL4 (20 ng/ml) for 8 h. The cells were visualized under microscope at 20× magnification.
Modulation of transcriptional activation of NF-κB
We then investigated the activation of the key transcription factor - NF-κB, a regulator for iNOS expression. When macrophages were pretreated with Hz and then challenged with IFNγ, there was a gradual increase in phosphorylation rate with the highest amount of phosphorylation of NF-κB at 60 minutes in comparison with IFNγ alone. However, we observed an opposite effect when macrophages were pretreated with Hz and then challenged with LPS. Initially there was phosphorylation of NF-κB at 15 minutes, but then the rate of phosphorylation decreases in comparison to LPS at 60 minutes (Figure 4). This transcriptional activity of NF-kB may relate to the result that is observed in Figure 2. These results indicate that Hz in some ways exerts differential expression of iNOS in combinatorial response to hemozoin and pro-inflammatory mediators by regulating the activation of transcription factor NF- κB. What molecular events cause this phenomenon and how it affects the iNOS expression is for further investigation.
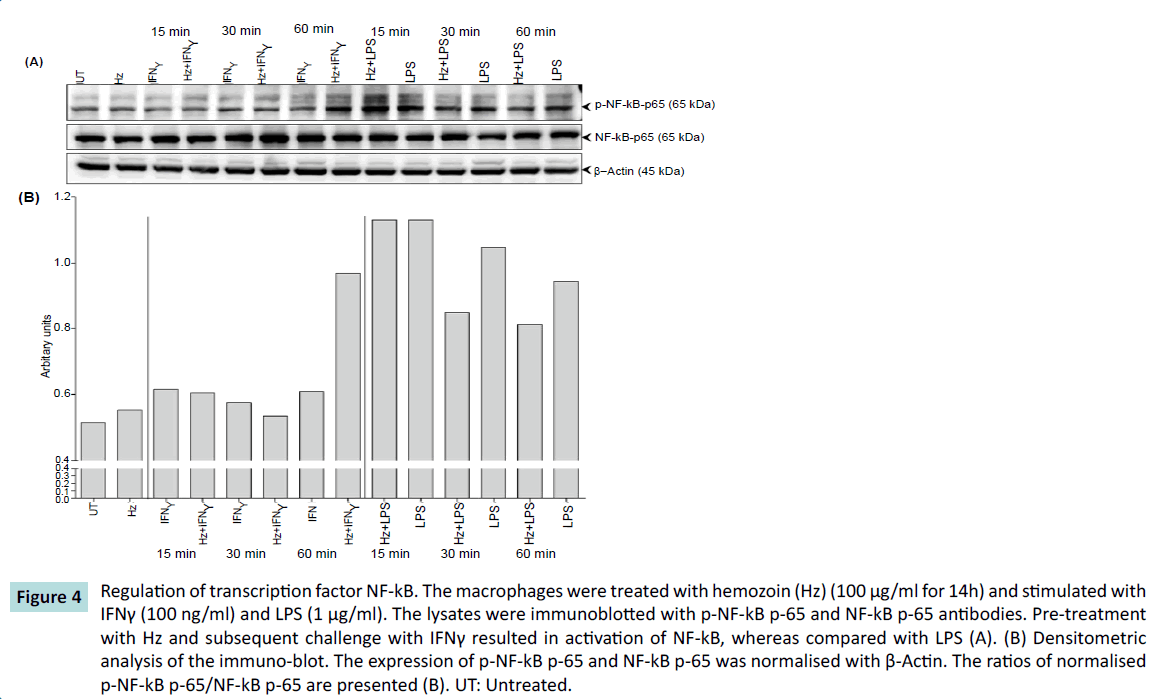
Figure 4: Regulation of transcription factor NF-kB. The macrophages were treated with hemozoin (Hz) (100 μg/ml for 14h) and stimulated with IFNγ (100 ng/ml) and LPS (1 μg/ml). The lysates were immunoblotted with p-NF-kB p-65 and NF-kB p-65 antibodies. Pre-treatment with Hz and subsequent challenge with IFNγ resulted in activation of NF-kB, whereas compared with LPS (A). (B) Densitometric analysis of the immuno-blot. The expression of p-NF-kB p-65 and NF-kB p-65 was normalised with β-Actin. The ratios of normalised p-NF-kB p-65/NF-kB p-65 are presented (B). UT: Untreated.
Conclusion
The results demonstrate hemozoin and inflammatory mediators exerts differential iNOS expression in macrophages. Additionally, based on our results, and previous publications, it is evident that different sources for acquiring hemozoin (natural, synthetic or commercial) may be confounding factors and may result in different experimental outcomes, and thus experiments should be judicially planned.
Conflict of Interest/Disclosures
The authors associated with this manuscript declare that there is no conflict of interest.
Author Contributions
Conceived and designed experiments: JWC, RR. Performed experiments: RR. Analyzed data: JWC RR. Contributed reagents/ materials/analysis tools: AHC. Wrote paper: JWC, RR. Edited the manuscript: JWC, RR, MK, AR.
Acknowledgment
Supported by NIH R01 HL075557 and HL068610 to JWC.
References
- World Health Organization (2015) Malaria Fact sheet N°94.
- HemmerCJ,Vogt A,Unverricht M, Krause R,Lademann M, et al. (2008)Malaria and bacterial sepsis: similar mechanisms of endothelial apoptosis and its prevention in vitro. Critical Care Medicine 36: 2562-2568.
- Auma MA,Siedner MJ,Nyehangane D,Nalusaji A (2013) Malaria is an uncommon cause of adult sepsis in south-western Uganda. Malar J 12:146.
- Franklin BS,Parroche P,Ataide MA,Lauw F,Ropert C, et al. (2009) Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci USA 106: 5789-5794.
- Egan TJ (2008) Haemozoin formation. Mol Biochem Parasitol 157: 127-136.
- Wunderlich F, Al-Quraishy S,Dkhil MA (2014) Liver-inherent immune system: its role in blood-stage malaria.Front Microbiol 5: 559.
- Bilzer M, Roggel F, Gerbes AL (2006) Role of Kupffer cells in host defense and liver disease. Liver International: Official Journal of The International Association for The Study of the Liver 26: 1175-1186.
- Boura M,Frita R, Gois A, Carvalho T,Hanscheid T (2013) The hemozoin conundrum: is malaria pigment immune-activating, inhibiting, or simply a bystander? Trends Parasitol 29:469-476.
- Deroost K, Tyberghein A, Lays N, Noppen S, Schwarzer E, et al. (2013) Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. Am J Respir Cell Mol Biol 48:589-600.
- Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U (2007) Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature.PLoS pathogens 3: e171.
- Dostert C, Guarda G, Romero JF, Menu P, Gross O, et al. (2009) Malarial hemozoin is a Nalp3 inflammasome activating danger signal.PloS one 4:e6510.
- Coban C, Yagi M, Ohata K, Igari Y, Tsukui T, et al. (2010)The malarial metabolite hemozoin and its potential use as a vaccine adjuvant. Allergology International : Official Journal of The Japanese. Society of Allergology 59: 115-124.
- Shio MT,Eisenbarth SC, Savaria M,Vinet AF,Bellemare MJ,et al. (2009) Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases.PLoS pathogens 5:e1000559.
- Skorokhod OA, Schwarzer E, Ceretto M, Arese P (2007) Malarial pigment haemozoin, IFN-gamma, TNF-alpha, IL-1beta and LPS do not stimulate expression of inducible nitric oxide synthase and production of nitric oxide in immuno-purified human monocytes.Malar J 6:73.
- Fang FC,Vazquez-Torres A (2002) Nitric oxide production by human macrophages: there's NO doubt about it. Am J Physiol Lung Cell Mol Physiol 282:941-943.
- Pitt BR, St. Croix CM (2002) Complex regulation of iNOS in lungs.Am J Respir Cell Mol Biol 26:6-9.
- MacMicking J, Xie QW,Nathan C (1997) Nitric oxide and macrophage function.Annu Rev Immunol 15:323-350.
- Ranjan R,Lee YG,Karpurapu M,Syed MA,Chung S,et al. (2015) p47phox and reactive oxygen species production modulate expression of microRNA-451 in macrophages.Free Radical Research 49:25-34.
- Ranjan R,Deng J, Chung S, Lee YG, Park GY,et al. (2014) The transcription factor nuclear factor of activated T cells c3 modulates the function of macrophages in sepsi.Journal of Innate Immunity 6:754-764.
- Shio MT,Kassa FA,Bellemare MJ,Olivier M (2010) Innate inflammatory response to the malarial pigment hemozoin.Microbes Infect12: 889-899.
- Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, et al. (2007)Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9.Proc Natl Acad Sci USA 104:1919-1924.
- Jaramillo M,Bellemare MJ, Martel C, Shio MT, Contreras AP, et al. (2009) Synthetic Plasmodium-like hemozoin activates the immune response: a morphology- function study. PloS One4: e6957.





