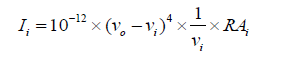Keywords
FT-IR; FT-Raman; TED; NBO; T2CNH
Introduction
Generally, Pyridine ring is a heterocyclic organic compound with the chemical formula C5H5N. Pyridine is structurally related to benzene, with one methine group (=CH-) replaced by a nitrogen atom. It occurs in many important compounds, including azines and the vitamins niacin and pyridoxal. The precursor of pyridine is used to agrochemicals, pharmaceuticals and is also an important solvent and reagent. Mostly, it is used in the in vitro synthesis of DNA, sulfa pyridine (a drug against bacterial and viral infections), antihistaminic drugs tripelennamine and mepyramine, as well as water repellents, bactericides, and herbicides. Some chemical compounds, although not synthesized from pyridine, contain its ring structure. They include B vitamins niacin and pyridoxal, an anti-tuberculosis drug isoniazide, nicotine and other nitrogencontaining plant products [1].
The ring of Thiophene and its derivatives have been reported to possess broad spectrum of biological properties including anti-inflammatory, analgesic, antidepressant, antimicrobial and anticonvulsant activities [2-4]. Antiepileptic drugs (AEDs) like tiagabine, etizolam, brotizolam are containing thiophene moiety in their structures as active pharmacophore [5,6]. In addition, thiophene and its derivatives functionalized with the formyl group are versatile building blocks for the synthesis of donor– acceptor substituted p-conjugated systems for several optical applications.
The hydrazone group in the organic compound brings out several physical and chemical properties. The hydrazones are bearing the >C=N-N< which leads the molecule towards nucleophilic and electrophilic in nature. In the hydrazone moiety, the nitrogen atom behaves as nucleophilic and carbon atom behaves as nucleophilic as well as electrophilic in nature [7-9]. The benzohydrazide derivatives shows wide spectrum of biological activities such as antibacterial [10], antifungal [11] and antitubercular [12] activities.
Subashchandrabose et al. [13] recorded the FT-IR, FT-Raman and UV-Vis spectra for the molecule N1-N2-bis((pyridine-4-l) methylene)benzene-1,2-diamine. The observed FT-IR and FTRaman spectral values were compared with the calculated wave numbers. For the prediction of accurate vibrational assignments TED analysis was performed using SQM method. The bond lengths and bond angles of stable conformer were correlated well with the experimental values. The hyperconjugative interaction and charge delocalization around the bonds were studied using NBO analysis. Band gap energy was also determined.
Quantum chemical calculations of energies, geometrical structure and vibrational wavenumber of 1,2-bis(3-methoxy-4- hydroxybenzylidene)hydrazine were carried out by Subramanian et al. [14] using DFT method with 6-31G(d) as basis set. The optimized geometrical parameters obtained by DFT calculations are in good agreement with single crystal XRD data. The vibrational spectral data were obtained from FT-IR and FT-Raman spectra are assigned based on the results of the theoretical calculations in solid phase.
From Literature survey reveals that the vibrational analysis of (E)-N′-(thiophen-2-ylmethylene) nicotinohydrazide (T2CNH) has not yet been reported. The T2CNH molecule was synthesized and its structural characterization was calculated by B3LYP/6- 311++G(d,p) basis set. The spectral investigation such as FT-IR, FTRaman and UV-Vis spectra were recorded. The observed spectral results were compared with the computed wavenumber. The vibrational assignments of the title molecule were carried out with the help of TED. The First order hyperpolarizability, Homo- Lumo energy gap was calculated and furthermore, the MEP and thermodynamic properties were also calculated.
Computational Details
The entire theoretical calculations were performed DFT method at B3LYP/6-311++G(d,p) basis set to using Gaussian 03W [15] program package, invoking gradient geometry optimization [15,16]. The geometrical parameters were used in the vibrational frequency calculations at the same level to characterize all the stationary points as minima. The vibrationally averaged nuclear positions of T2CNH were used for harmonic vibrational frequency calculations resulting in IR and Raman frequencies together with intensities and Raman depolarization ratios. The vibrational modes were assigned on the basis of TED analysis using VEDA4 program package [17]. The Raman activity was calculated by using Gaussian 03W package and the activity was transformed into Raman intensity using Raint program [18] by the expression:
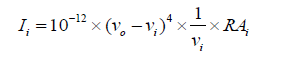
Where Ii is the Raman intensity, RAi is the Raman scattering activities, νi is the wavenumber of the normal modes and ν0 denotes the wavenumber of the excitation laser [19].
Experimental Details
Synthesis procedure
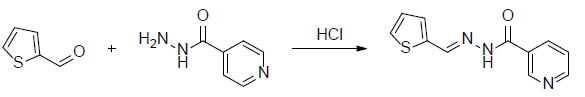
A mixture of Thiophene-2-carboxaldehyde (2.1 mL, 0.01 mol) and nicotinic acid hydrazide (1.37 g, 0.01 mol) in 5 mL of ethanol was stirred in the presence of 2 drops of concentrated HCl for one hour. The reaction mixture was maintained at room temperature and the colourless solid was obtained. The solid was separated and filtered under suction, washed with ice-cold water (50 ml). The precipitate was washed with water and filtered and again washed with petroleum ether (40-60%) and dried over in a vacuum desicator then the product was recrystallized from hot ethanol.
Results and Discussion
Molecular Geometry
The T2CNH molecule was optimized by B3LYP/6-311++G(d,p) level of basis set. This molecule consists of thiophene and pyridine rings are fused by hydrazone linkage. The calculated bond length of C2-S1 is expected to be shorter than the C5-S1 bond length, since the hydrozone moiety is attached with C2 atom and their corresponding bond distances are 1.750 and 1.728Å. The bond lengths of C2=C3, C3-C4, C4=C5 are 1.377, 1.418, 1.369Å and the bond angles of C2-C3-C4, C3-C4-C5 are 113.02°, 113.03°. The bond angles S1-C2-C3 and S1-C5-C4 are differ by ~1° which is due to the shortening of bond distance between S1 and C2 atoms. These values are particularly in good agreement with the values of anti thiophen-2-aldehyde [20] and thiophen- 2-carbohydrazide [21]. Furthermore, these values are also find support from literature [22]. The dihedral angles of S1-C2-C9-N11/ S1-C2-C9-H10 and C3-C2-C9-N11/C3-C2-C9-H10 are -179.96°/-0.07° and 0.03°/+179.93°, respectively, which shows that the thiophen and hydrozone moieties of T2CNH are co-planar. Since there is a good conjugation between p-orbitals of all atoms of thiophen and hydrazone moieties. Most of the calculated geometrical parameters are find support from single crystal X-ray diffraction values [23,24]. It should be mentioned that there is no significant difference between the geometrical parameters of the hydrozone and pyridine moieties [23,24]. The geometrical parameters and optimized structure of the T2CNH molecule are presented in Table 1 and Figure 1, respectively.
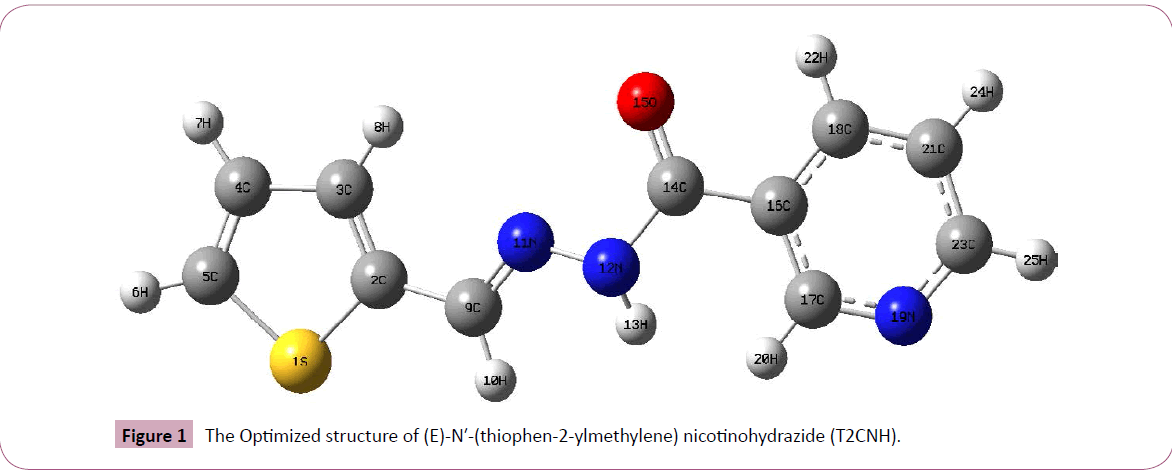
Figure 1: The Optimized structure of (E)-N′-(thiophen-2-ylmethylene) nicotinohydrazide (T2CNH).
| Bond Parametres |
B3LYP/6-311++G(d,p) |
XRDa,b,c |
| Bond Lengths (Å) |
| S1-C2 |
1.750 |
1.728b |
| S1-C5 |
1.728 |
1.717a, 1.707b |
| C2-C3 |
1.377 |
1.375a, 1.362b |
| C2-C9 |
1.448 |
|
| C3-C4 |
1.418 |
1.431a, 1.392b |
| C3-H8 |
1.081 |
1.114a |
| C4-C5 |
1.369 |
1.345b |
| C9-H10 |
1.097 |
0.930c |
| C9-N11 |
1.282 |
1.266c |
| N11-N12 |
1.357 |
1.375c |
| N12-H13 |
1.016 |
0.860c |
| N12-C14 |
1.385 |
1.354c |
| C14-O15 |
1.213 |
1.224c |
| C16-C17 |
1.399 |
1.384c |
| C16-C18 |
1.397 |
1.3714c |
| C17-N19 |
1.335 |
1.3344c |
| C17-H20 |
1.087 |
0.9304c |
| C18-C21 |
1.387 |
1.3684c |
| C18-H22 |
1.083 |
0.9304c |
| N19-C23 |
1.335 |
1.3374c |
| C21-C23 |
1.395 |
1.3684c |
| C23-H25 |
1.086 |
0.9304c |
| Bond Angles (°) |
|
|
| C2-S1-C5 |
91.57 |
92.00a, 91.81b |
| S1-C2-C3 |
110.68 |
111.80a |
| C2-C3-C4 |
113.02 |
112.20a, 114.72b |
| C2-C3-H8 |
122.16 |
129.20a |
| C3-C4-C5 |
113.03 |
112.05b |
| S1-C5-C4 |
111.68 |
112.13b |
| N12-C14-C16 |
114.21 |
114.95c |
| O15-C14-C16 |
122.13 |
121.48c |
| C16-C17-N19 |
123.88 |
122.05c |
| C16-C17-H20 |
120.91 |
120.03c |
| N19-C17-H20 |
115.16 |
118.20c |
| C16-C18-C21 |
118.94 |
118.91c |
| C16-C18-H22 |
119.08 |
120.50c |
| C21-C18-H22 |
121.96 |
120.30c |
| C17-N19-C23 |
117.47 |
117.44c |
| C18-C21-C23 |
118.58 |
118.58c |
| C18-C21-H24 |
121.13 |
120.71c |
| C23-C21-H24 |
120.27 |
120.70c |
| N19-C23-C21 |
123.40 |
123.64c |
| Dihedral Angles (°) |
|
|
| S1-C2-C9-H10 |
-0.07 |
|
| S1-C2-C9-N11 |
-179.96 |
|
| C3-C2-C9-H10 |
179.93 |
|
| C3-C2-C9-N11 |
0.03 |
|
| C2-C3-C4-C5 |
0.02 |
|
| C2-C3-C4-H7 |
-179.98 |
|
| H8-C3-C4-C5 |
179.97 |
|
| H8-C3-C4-H7 |
-0.03 |
|
| C3-C4-C5-S1 |
-0.01 |
|
| C3-C4-C5-H6 |
-179.99 |
|
| H7-C4-C5-S1 |
179.99 |
|
| H7-C4-C5-H6 |
0.01 |
|
| C2-C9-N11-N12 |
179.45 |
|
| H10-C9-N11-N12 |
-0.43 |
|
| C9-N11-N12-H13 |
1.93 |
|
| C9-N11-N12-C14 |
175.56 |
|
| N11-N12-C14-O15 |
-2.82 |
|
| N11-N12-C14-C16 |
177.89 |
|
| H13-N12-C14-O15 |
170.80 |
|
| H13-N12-C14-C16 |
-8.47 |
|
| N12-C14-C16-C17 |
-28.41 |
|
| N12-C14-C16-C18 |
154.27 |
|
| O15-C14-C16-C17 |
152.29 |
|
| O15-C14-C16-C18 |
-25.01 |
|
| C14-C16-C17-N19 |
-178.88 |
|
| C14-C16-C17-H20 |
-0.97 |
|
| C18-C16-C17-N19 |
-1.57 |
|
aBrathenO, KvesethK, NielsenKJ, HagenK (1986) J MolStruct 145: 45.
bGeigerDK, GeigerHC, WilliamsL, NollBC (2012) ActaCrystE 68 : 0420.
cRameshBabuN (2014) J MolStruct 1072: 84-93.
Table 1: The optimized bond parameters of T2CNH.
Vibrational Assignments
The T2CNH molecule belongs to C1 point group symmetry. It consists of 25 atoms which undergoes 69 normal modes of vibrations. In which 47 modes of vibrations are in-plane and 22 are out-of-plane bending vibrations and all of them are IR and Raman active [25]. The calculated and observed vibrational wavenumbers using DFT/B3LYP/6-311++G(d,p) basis set, along with their relative intensities are given in Table 2. The total energy distribution (TED) was calculated using SQM program [26]. The combined vibrational spectra of T2CNH molecule are shown in Figures 2 and 3.
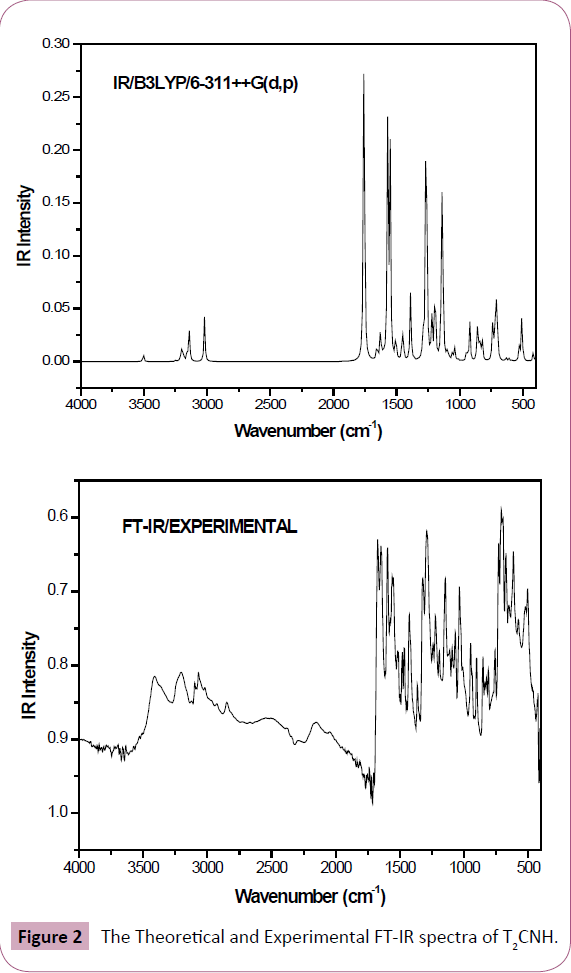
Figure 2: The Theoretical and Experimental FT-IR spectra of T2CNH.
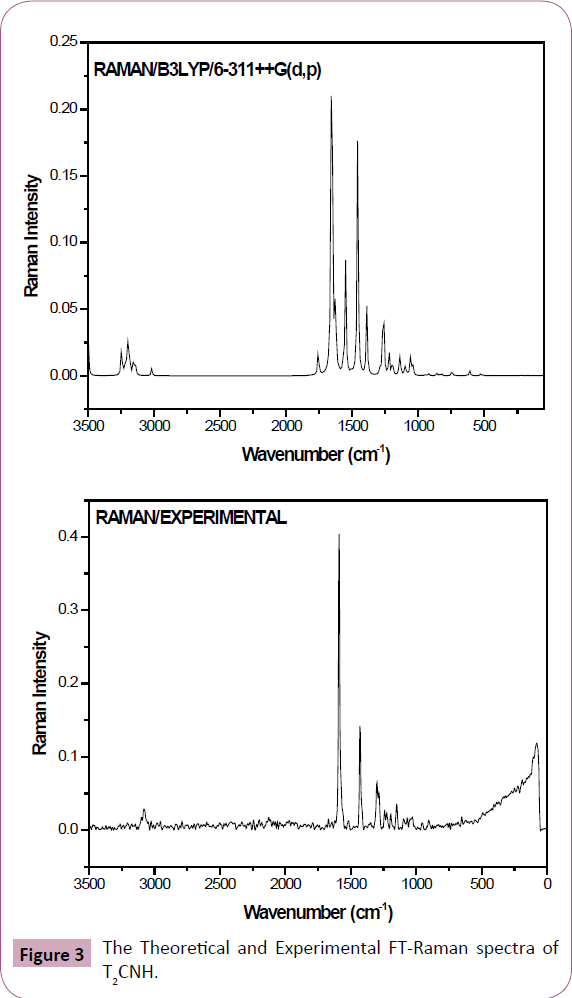
Figure 3: The Theoretical and Experimental FT-Raman spectra of T2CNH.
N12(34)
Mode
No |
Calculated Frequencies (cm-1) |
Observed Frequencies (cm-1) |
IR Intensity |
Raman Intensity |
Reduced
Masses |
Force
Consts |
Vibrational assignments≥10% (TED)d |
| Un Scaled |
Scaleda |
FT-IR |
FT-Raman |
Abs. |
Rel.b |
Abs. |
Rel.c |
| 1 |
3503 |
3365 |
3401 w |
|
7.42 |
2.06 |
134.41 |
1.67 |
1.08 |
7.78 |
νN12H13(100) |
| 2 |
3247 |
3120 |
3171 w |
|
1.23 |
0.34 |
101.27 |
1.26 |
1.10 |
6.82 |
νC5H6(94) |
| 3 |
3223 |
3096 |
|
|
0.57 |
0.16 |
34.50 |
0.43 |
1.10 |
6.70 |
νC3H8(97) |
| 4 |
3204 |
3078 |
|
3079 w |
6.29 |
1.75 |
62.54 |
0.78 |
1.09 |
6.59 |
νC4H7(96) |
| 5 |
3202 |
3076 |
|
|
7.00 |
1.94 |
72.48 |
0.90 |
1.10 |
6.62 |
νC18H22(99) |
| 6 |
3187 |
3062 |
3059 w |
|
9.14 |
2.54 |
84.20 |
1.05 |
1.09 |
6.53 |
νC21H24(95) |
| 7 |
3156 |
3032 |
|
|
10.92 |
3.03 |
72.05 |
0.89 |
1.09 |
6.39 |
νC23H25(92) |
| 8 |
3139 |
3016 |
|
|
29.24 |
8.12 |
29.47 |
0.37 |
1.09 |
6.32 |
νC17H20(99) |
| 9 |
3019 |
2901 |
|
|
42.64 |
11.84 |
29.11 |
0.36 |
1.09 |
5.83 |
νC9H10(100) |
| 10 |
1757 |
1688 |
1673 s |
1670 w |
360.16 |
100.00 |
392.18 |
4.87 |
11.08 |
20.16 |
νO15C14(85) |
| 11 |
1656 |
1591 |
1592 s |
1589 s |
15.73 |
4.37 |
8057.31 |
100.00 |
7.23 |
11.68 |
νN11C9(75)+βH10C9N11(20) |
| 12 |
1627 |
1563 |
|
|
32.79 |
9.10 |
1339.53 |
16.63 |
5.75 |
8.96 |
νC18C21(30)+νN19C23(30)+βC21C23N19(25) |
| 13 |
1605 |
1542 |
1549 w |
|
6.21 |
1.73 |
25.40 |
0.32 |
5.21 |
7.91 |
νC21C23(31)+νC16C18(27)+βC17N19C23(10) |
| 14 |
1570 |
1508 |
|
1519 w |
217.56 |
60.41 |
126.25 |
1.57 |
3.38 |
4.91 |
νC2C3(28)+βH13N12N11(22)+νC4C5(13)+νC2C9(11) |
| 15 |
1551 |
1490 |
|
|
202.85 |
56.32 |
2133.94 |
26.48 |
2.19 |
3.10 |
νC2C3(14)+βH13N12N11(41)+νC4C5(12) |
| 16 |
1506 |
1447 |
1425 m |
1429 m |
26.23 |
7.28 |
58.60 |
0.73 |
2.15 |
2.87 |
βH24C21C23(27)+βH20C17C16(25) |
| 17 |
1459 |
1402 |
|
|
8.66 |
2.41 |
4817.87 |
59.80 |
4.83 |
6.07 |
νC2C3(13)+νC4C5(32)+βH7C4C5(14)+νC3C4(15) |
| 18 |
1448 |
1391 |
|
|
26.37 |
7.32 |
62.23 |
0.77 |
2.18 |
2.69 |
βH25C23N19(43)+βC16C17N19(23) |
| 19 |
1390 |
1335 |
|
|
52.04 |
14.45 |
1478.54 |
18.35 |
1.75 |
1.99 |
νC4C5(13)+βH10C9N11(38)+βH6C5C4(13) |
| 20 |
1387 |
1333 |
|
|
16.34 |
4.54 |
60.17 |
0.75 |
2.08 |
2.36 |
βH10C9N11(25)+βH6C5C4(15)+νC3C4(18) |
| 21 |
1362 |
1308 |
1290 s |
1300 m |
0.98 |
0.27 |
2.27 |
0.03 |
1.31 |
1.43 |
βH20C17C16(45)+βH22C18C21(23) |
| 22 |
1291 |
1240 |
|
1241 w |
19.71 |
5.47 |
141.74 |
1.76 |
7.28 |
7.15 |
νN19C23(43)+νC16C18(27) |
| 23 |
1268 |
1219 |
1218 m |
1225 w |
94.23 |
26.16 |
45.69 |
0.57 |
1.54 |
1.46 |
βH7C4C5(23)+βH8C3C4(29)+βH6C5C4(13) |
| 24 |
1265 |
1215 |
|
|
221.71 |
61.56 |
2432.18 |
30.19 |
2.39 |
2.25 |
νC14C16(22) |
| 25 |
1221 |
1173 |
|
1195 w |
39.47 |
10.96 |
610.76 |
7.58 |
1.60 |
1.40 |
νC18C21(11)+βH24C21C23(17)+βH25C23N19(30) |
| 26 |
1195 |
1148 |
1145 m |
1149 w |
87.67 |
24.34 |
456.90 |
5.67 |
3.39 |
2.85 |
νN11N12(60) |
| 27 |
1142 |
1097 |
|
1096 w |
146.68 |
40.73 |
527.62 |
6.55 |
2.70 |
2.07 |
νC2C9(10)+νN12C14(40) |
| 28 |
1134 |
1090 |
|
|
69.91 |
19.41 |
242.18 |
3.01 |
1.45 |
1.10 |
βH24C21C23(20)+βC16C17N19(12)+βH22C18C21(36) |
| 29 |
1102 |
1059 |
|
|
7.47 |
2.07 |
295.96 |
3.67 |
1.26 |
0.90 |
νC4C5(15)+βH7C4C5(22)+βH6C5C4(47) |
| 30 |
1086 |
1044 |
|
|
5.12 |
1.42 |
134.46 |
1.67 |
3.17 |
2.21 |
νN11N12(24)+νN12C14(16) |
| 31 |
1058 |
1017 |
1031 w |
1030 w |
1.76 |
0.49 |
393.20 |
4.88 |
3.19 |
2.11 |
βC16C18C21(45) |
| 32 |
1056 |
1015 |
|
|
7.59 |
2.11 |
414.19 |
5.14 |
1.76 |
1.15 |
βH7C4C5(14)+βH8C3C4(27)+νC3C4(35) |
| 33 |
1039 |
999 |
|
|
11.95 |
3.32 |
318.45 |
3.95 |
4.30 |
2.73 |
βC17N19C23(32)+βC21C23N19(15)+βC18C21C23(25) |
| 34 |
1014 |
974 |
|
|
2.23 |
0.62 |
6.67 |
0.08 |
1.38 |
0.83 |
τH22C18C21H24(75)+ΓC23C21N19H25(18) |
| 35 |
987 |
949 |
948 w |
955 w |
0.58 |
0.16 |
8.33 |
0.10 |
1.43 |
0.82 |
ΓC23C21N19H25(50)+ΓC17C16N19H20(12)+ΓC18C16C21H22(30) |
| 36 |
947 |
910 |
|
|
1.90 |
0.53 |
6.99 |
0.09 |
1.39 |
0.73 |
ΓC17C16N19H20(71) |
| 37 |
945 |
908 |
|
904 w |
9.84 |
2.73 |
50.68 |
0.63 |
1.50 |
0.79 |
ΓH10C9N11H12(80) |
| 38 |
928 |
891 |
897 w |
|
0.39 |
0.11 |
6.15 |
0.08 |
1.38 |
0.70 |
τH6C5C4H7(46)+ΓC3C2C4H8(32) |
| 39 |
921 |
885 |
|
|
38.10 |
10.58 |
86.38 |
1.07 |
7.12 |
3.56 |
βN12C14O15(20)+βC14N12N11(25) |
| 40 |
861 |
827 |
846 w |
|
31.22 |
8.67 |
114.39 |
1.42 |
4.31 |
1.88 |
βC4C5S1(80) |
| 41 |
851 |
817 |
|
|
8.44 |
2.34 |
1.04 |
0.01 |
1.29 |
0.55 |
τH6C5C4H7(36)+ΓC3C2C4H8(55) |
| 42 |
841 |
808 |
|
|
13.98 |
3.88 |
48.48 |
0.60 |
1.97 |
0.82 |
τH22C18C21H24(17)+ΓC23C21N19H25(12)+ΓC18C16C21H22(32)+ΓO15C16N12C14(12) |
| 43 |
823 |
791 |
|
|
25.89 |
7.19 |
107.27 |
1.33 |
4.43 |
1.77 |
νC2C3(10)+βC2C9N11(20)+νS1C2(17) |
| 44 |
747 |
718 |
|
|
3.22 |
0.89 |
221.87 |
2.75 |
7.15 |
2.35 |
βC2C3C4(57)+νS1C2(23) |
| 45 |
739 |
710 |
700 w |
|
31.92 |
8.86 |
64.22 |
0.80 |
2.76 |
0.89 |
ΓC18C16C21H22(25)+ΓO15C16N12C14(50) |
| 46 |
724 |
695 |
|
|
21.84 |
6.06 |
20.02 |
0.25 |
4.31 |
1.33 |
βC21C23N19(17)+τC16C17C23N19(16)+τC16C18C23C21(16)+τC18C21N19 C23(10) |
| 47 |
718 |
690 |
|
|
16.37 |
4.54 |
7.30 |
0.09 |
3.96 |
1.20 |
τC16C17C23N19(27)+τC16C18C23C21(22)+τC18C21N19 C23(16) |
| 48 |
706 |
679 |
|
652 w |
75.61 |
20.99 |
10.36 |
0.13 |
1.17 |
0.34 |
τH6C5C4H7(13)+τH6C5S1C2(81) |
| 49 |
633 |
608 |
607 s |
|
4.00 |
1.11 |
49.48 |
0.61 |
7.72 |
1.82 |
βC16C17N19(15)+βC17N19 C23(15)+βC18C21C23(41) |
| 50 |
610 |
586 |
|
|
2.58 |
0.72 |
360.42 |
4.47 |
9.05 |
1.98 |
βC2S1C5(63) |
| 51 |
586 |
563 |
|
|
1.22 |
0.34 |
16.70 |
0.21 |
3.25 |
0.66 |
τC3C2C4H5(79) |
| 52 |
528 |
507 |
503 m |
|
15.76 |
4.38 |
167.78 |
2.08 |
3.01 |
0.49 |
βC16C14N12(22)+τH13N12C14C16(22)+ΓC14C16C18C17(10) |
| 53 |
508 |
488 |
|
|
44.93 |
12.47 |
72.78 |
0.90 |
1.60 |
0.24 |
τH13N12C14C16(61) |
| 54 |
492 |
472 |
|
|
0.31 |
0.09 |
8.91 |
0.11 |
4.01 |
0.57 |
τC2S1C4C5(65) |
| 55 |
451 |
433 |
|
|
0.46 |
0.13 |
24.54 |
0.30 |
7.97 |
0.95 |
βC2C9N11(14)+νS1C2(12)+βC17,C16C18(10)+βC9C2S1 (12)+βC14N12N11(11) |
| 56 |
419 |
402 |
|
|
7.99 |
2.22 |
9.91 |
0.12 |
4.68 |
0.48 |
τC16C17C23N19(15)+τC16C18C23C21(31) |
| 57 |
396 |
381 |
|
|
4.89 |
1.36 |
11.97 |
0.15 |
3.36 |
0.31 |
τC16C18C23C21(16)+τC18C21N19C23(34)+ΓC14C16C18C17(10) |
| 58 |
379 |
364 |
|
|
1.32 |
0.37 |
1.86 |
0.02 |
8.03 |
0.68 |
νC14C16(17)+βN12C14O15(22)+βC17C16C18(20) |
| 59 |
340 |
326 |
|
|
1.88 |
0.52 |
29.62 |
0.37 |
5.99 |
0.41 |
τC2S1C4C5(18)+ τC2C9N11 |
|
|
|
| 60 |
264 |
253 |
|
|
13.84 |
3.84 |
34.50 |
0.43 |
4.73 |
0.19 |
βC14C16C18(36) |
| 61 |
226 |
217 |
|
224 w |
11.59 |
3.22 |
71.81 |
0.89 |
3.69 |
0.11 |
τC5S1C2C9(11)+ΓC16C14N12N11(20)+τC3C2C9N11(16) |
| 62 |
213 |
205 |
|
191 w |
1.15 |
0.32 |
136.24 |
1.69 |
10.18 |
0.27 |
βC9 C2S1(30)+βC14N12N11(22) |
| 63 |
170 |
163 |
|
|
11.98 |
3.33 |
135.90 |
1.69 |
4.69 |
0.08 |
τC9 N11N12C14(20)+ΓC14C16C18C17(14)+τC3C2C9N11(13) |
| 64 |
130 |
125 |
|
|
1.52 |
0.42 |
83.59 |
1.04 |
5.74 |
0.06 |
τC9N11N12zC14(25)+τC5S1C2C9 (37)+ΓC14C16C18C17(10) |
| 65 |
120 |
115 |
|
|
6.55 |
1.82 |
60.10 |
0.75 |
5.71 |
0.05 |
βC2C9N11(18)+βC16C14N12(18)+βC9C2S1(17)+ΓC14C16C18C17(14) |
| 66 |
64 |
61 |
|
80 m |
5.89 |
1.63 |
381.03 |
4.73 |
5.90 |
0.01 |
τC5 S1C2C9(12)+τC18 C16C14N12(45)+τC3C2C9N11(15) |
| 67 |
46 |
45 |
|
|
0.73 |
0.20 |
161.56 |
2.01 |
6.68 |
0.01 |
βC2C9N11(16)+βC16C14N12(14)+βC9N11N12(20)+βC14N12N11(22) |
| 68 |
32 |
31 |
|
|
1.86 |
0.52 |
329.50 |
4.09 |
5.69 |
0.00 |
τC2 C9N11N12(18)+τC18C16C14N12(15)+τC16 C14N12N11(49) |
| 69 |
27 |
26 |
|
|
0.47 |
0.13 |
967.33 |
12.01 |
5.46 |
0.00 |
τC9 N11N12C14(18)+τC18 C16C14N12(25)+τC3 C2C9N11(18) |
ν: Stretching, β: in-plane-bending, Γ: out-of-plane bending, τ- Torsion, vw: very week, w:week, m:medium, s:strong, vs: very strong.
aScaling factor: 0.9608.
bRelative IR absorption intensities normalized with highest peak absorption equal to 100.
cRelative Raman intensities calculated by Equation (1) and normalized to 100.
dTotal energy distribution calculated at B3LYP/6-311++G(d,p) level.
Table 2: The experimental and calculated frequencies of T2CNH using B3LYP/6-311++G(d,p) level of basis set [harmonic frequencies (cm−1), IR, Raman intensities (Km/mol), reduced masses (amu) and force constants (mdynA°−1)].
N-H vibrations
The N-H stretching vibration appears in the region of 3300-3500 cm-1 [27]. In accordance with the above literature the νN-H vibration has been observed at 3401 cm-1 in FTIR spectrum, whereas the harmonic wavenumber assigned at 3365 cm-1 (mode no: 1) and its TED value is 100% it should be noted here that, a small deviation between theoretical and experimental value is only due to intra-molecular charge transfer between amino and carbonyl group in hydrazone linkage. The harmonic wavenumbers (1508/ mode no: 14 and 488 cm-1/mode no: 53) of βN-H and ΓN-H modes were presented in Table 2 are found to be in good agreement with literature [28] data 1476 and 535 cm-1 as well as with the structurally related molecule. Mode no: 14 is in agreement with observed FT-Raman band at 1519 cm-1 and these assignments also find support from TED values (22% and 61%).
C-H vibrations
In aromatic compounds, the νCH, βCH and ΓCH vibrations are expected to appear in the ranges of 3100-3000 cm-1, 1400-1000 cm-1 and 1000-750 cm-1, respectively [29,30]. In this study, the aromatic C-H stretching vibration is observed at 3059 cm-1 /FT-IR, whereas the calculated wavenumbers are in the range 3076-3016 cm-1 (mode nos. 5 and 9). The in-plane C-H bending vibrations are assigned at 1425, 1290 cm-1 in FT-IR and 1429, 1300 & 1195 cm-1 in FT-Raman spectra and their corresponding calculated wavenumbers are 1447, 1391, 1308, 1173 cm-1 (mode nos: 16, 18, 21, 25). The harmonic wavenumbers occur in the region of 974-808 cm-1 (mode nos: 34-36, 42) are assigned to C-H out-ofplane bending modes, in which mode no: 35 is in agreement with observed wavenumber values: 955 cm-1 (FT-Raman) and 948 cm-1 (FT-IR). These assignments are in line with literature values [14] and also find support from TED Values.
The νCH modes are expected to appear in the range of 3100- 3000 cm-1 with multiple weak bands and the bands are not affected appreciably by the nature of the substituent’s [31,32]. The νC-H vibration of the thiophen ring is observed at 3171 cm-1 in FT-IR and 3079 cm-1 in FT-Raman spectra in accordance with the literature [20-22]. The harmonic values corresponding to νC-H are calculated in the range 3120-3078 cm-1 (mode nos: 2-4) with TED values >96%. This indicates that these are highly pure stretching modes. The βC-H vibrations appear by sharp but weak to medium bands in the range 1100-1500 cm-1 and the bands are not sensitive to the nature of substitutents. The ΓC-H deformation modes are expected to appear in the range 800-1000 cm-1 [32]. The IR active βC-H/ΓC-H vibrations of T2CNH molecule are observed at 1218/897 cm-1, respectively. The harmonic wavenumbers of these bands are 1219, 1059, 1015 and 891, 817 cm-1 in good agreement with literature values [20-22].
The harmonic frequencies for νC-H, βC-H and ΓC-H modes in hydrozone linkage were assigned at 2919, 1315 and 910 cm-1, respectively and these assignments are in line with assignments νC-H: 2901, βC-H: 1335, ΓC-H: 908 cm-1 (mode nos: 9, 19, 37) are made in the present study. These vibrational assignments are further supported by literature [28] and TED output.
C=C, C-C vibrations
In the pyridine ring, the ν(C-C) stretching vibrations are usually occur in the ranges of 1590-1640, 1560-1580 and 1470-1510 cm-1 [33]. The computed wavenumber for ν(C-C) modes are lies at 1563, 1542, 1240 and 1173 cm-1 (mode nos: 12, 13, 22 and 25) with TED values. In the present study it has been established well and the FTIR band at 1549 cm-1 and FT-Raman bands at 1241, 1195 cm-1 are designated as νC-C vibrations. These assignments are find support from the literature [34] and also from TED values.
The bands arising from βCCC and ΓCCC of pyridine moiety are ascribed to bands observed at 1031, 607 and 503 cm-1 respectively in FTIR spectrum. These vibrational assignments find support from harmonic bands: 1017, 999, 608 and 507, 381, 163 cm-1 (mode nos: 31, 33, 49 and 52, 57, 63) in addition to literature values [35].
The ring carbon-carbon stretching vibrations in thiophen ring are reported in the ranges of 1329-1431, 1420-1501 and 1419-1519 cm-1, respectively [20-22]. For the same mode the computed wavenumbers are: 1508 (C=C), 1490 (C=C) and 1402 cm-1 (C-C). These assignments are having considerable TED values ≥ 15% and its corresponding mode no: 14 is further supported by observed Raman band 1519 cm-1. The βCCC mode of thiophene moiety is ascribed to mode no: 44 (718 cm-1), this supported by TED value (57%) also. The mode nos: 27 and 24 are attributed to νC2-C9 and νC14-C16 mode and are in agreement with literature [28].
C=N, C-N vibrations
The identifications of C=N and C-N vibrations is a difficult task, since the mixing of vibrations is possible in this region [36]. However, with the help of Gauss View (3.0) software and TED results, those vibrations are described and assigned in this study. The C=N and C-N stretching vibrations appear in the ranges of 1670-1600 cm-1 and 1382-1266 cm-1 respectively [36]. In hydrozone linkage, the νC9=N11 and νC14-N12 stretching vibrations are assigned respectively at 1592 cm-1 (FTIR) /1589 cm-1 (FT-Raman) and at 1096 cm-1 (FT-Raman). The TED results show that these vibrations are mixed with βHCN and νC-C modes and their corresponding harmonic frequencies are 1591 (mode no: 11) and 1097 cm-1 (mode no: 27) well correlated with experimental observations. These vibrational assignments are also supported by literature [28] in addition to TED output (75% and 40%).
In pyridine ring, the harmonic/observed bands at 1563 (mode no: 12), 1240 (mode no: 22)/1241 cm-1 in FT-Raman spectrum can be assigned to C-N stretching vibration and TED also predict that these vibrations are mixed with νC-C modes. These assignments are in good agreement with our earlier study [28] and also find support from TED values (30%, 43%). The βC21-C23-N19, βC16-C17-N19, βC17-N19-C23 and βC2-C9=N11, βC16-C14-N12 deformations belong to pyridine and hydrozone moieties, respectively assigned to 1563, 1391, 999 and 791, 507 cm-1 (mode nos: 12, 18, 33 and 43, 52). Similarly the τC16C17C23N19, τC18C21N19C23, τC3C2C9N11, ΓC16C14N12N11 modes are assigned to wavenumbers: 690, 381, 217, 217 cm-1 (mode nos: 47, 57, 61, 61).
N-N vibrations
The νN-N stretching was observed at 1145 cm-1 in FTIR is undoubtedly assigned to νN11-N12 vibration and the value of this band is calculated at 1148 cm-1 (mode no: 26) with a TED of (60%) [28]. This assignment is well correlated with observed FT-Raman band 1149 cm-1. The in-plane bending vibrations of C2-C9=N11, C9=N11-N12 & C14-N12-N11 are assigned to wavenumbers: 791, 45 & 885, having considerable TED values (≥ 20%). The wavenumbers 163, 326 cm-1 (mode nos: 63, 59) are assigned to τC9=N11-N12-C14, τC2-C9=N11-N12 modes.
C-S vibrations
In T2CNH the scaled wavenumbers 791 and 718 cm-1 (mode nos: 43 and 44) are assigned to νC-S modes of thiophen ring moiety. This assignment is in agreement with the assignments proposed by various authors [20,21]. This mode is well known to mix with neighboring modes (νCC, βCCN/mode no: 43 and βCCC/mode no: 44) as reported in the literature [37]. The harmonic frequencies of βCSC and ΓCSC vibrations are ascribed to wavenumbers: 586 cm-1 (mode no: 50) and 125 cm-1 (mode no: 64) with 62% and 37% of TED values, respectively. Further the wavenumbers 827 cm-1 (mode no: 40) and 205 cm-1 (mode no: 62) are assigned to βC4C5S1 and βC9C2S1 modes, respectively, which are in line with the observed bands (846 cm-1: FTIR and 191 cm-1: FT-Raman) in addition to support the TED values [80% and 30%].
C=O vibrations
The C=O is formed by Pπ-Pπ bonding between carbon and oxygen atoms. Carbonyl (C=O) group stretching vibration is expected to appear in the region of 1680-1715 cm-1 [38]. In this study, the carbonyl group stretching vibration appear at 1670 cm-1 as strong band in FT-IR and at 1673 cm-1 as weak band in FT-Raman spectra. The values of νC=O band is calculated at 1688 cm-1 (mode no: 10) with a TED of 85%. The βC=O and ΓC=O vibrations are assigned respectively to mode nos: 39 and 45, in which the predicted wavenumber related to ΓC=O mode is found to be in moderate agreement with the observed FTIR band at 700 cm-1.
NLO Property
Analysis of organic compounds having conjugated π-electron systems and large hyperpolarizability using IR and Raman spectroscopy has evolved as a subject of scientific research. The application of the title molecule in the field of non-linear optics demands the investigation of its structural and bonding features contributing to the hyperpolarizability enhancement by analyzing the vibrational modes using IR and Raman spectroscopy. The DFT/ B3LYP/6-311++G(d,p) basis set has been used for the prediction of first hyperpolarizability.
The calculated first hyperpolarizability and the total molecular dipole moment of T2CNH is 1.5861 × 10−30 esu, 0.9906 Debye, respectively obtained by B3LYP/6-311++G(d,p) level of theory. The total dipole moment of the title molecule is moderately equal and β0 value of T2CNH is 4 times greater than that of urea, hence the molecule T2CNH has considerable non-linear optical (NLO) activity and the hyperpolarizabilities of T2CNH are given in Table 3.
| Parameters |
B3LYP/6-311++G(d,p) |
| Dipole moment ( μ ) Debye |
| μx |
0.3145 |
| μy |
-0.7701 |
| μz |
0.5380 |
| μ |
0.9906Debye |
| Polarizability ( α0 )× 10-30esu |
| αxx |
330.88 |
| αxy |
-3.11 |
| αyy |
168.52 |
| αxz |
-4.63 |
| αyz |
-0.11 |
| αzz |
97.39 |
| α0 |
0.5318x10-30esu |
| Hyperpolarizability( β0 )× 10-30esu |
| βxxx |
-1853.85 |
| βxxy |
229.60 |
| βxyy |
-33.45 |
| βyyy |
-129.96 |
| βxxz |
40.31 |
| βxyz |
21.11 |
| βyyz |
2.92 |
| βxzz |
54.46 |
| βyzz |
-29.90 |
| βzzz |
-38.55 |
| β0 |
1.5861x10-30esu |
Standard value for urea (μ=1.3732 Debye, β0=0.3728 × 10-30 esu): esu-electrostatic unit
Table 3: The NLO measurements of T2CNH.
NBO Analysis
This method gives information about the intra- and inter-molecular interactions among bonds. Furthermore, it provides a convenient basis for investigating the interactions in both filled and virtual orbital spaces along with charge transfer and conjugative interactions in molecular system [39]. The natural bonding orbital (NBO) analysis was performed for T2CNH using B3LYP/6- 311++G(d,p) basis set in order to elucidate the intra-molecular, hybridization and delocalization of ED within T2CNH. The strong intra-molecular hyperconjugative interaction of the σ and π electrons of C−C to the anti C−C bond of the pyridine ring leads to stabilization of some part of the pyridine ring. The NBO analysis has been carried out by B3LYP/6-311++G(d,p) basis set and the E(2) values and types of the transition are shown in Table 4.
| Type |
Donor NBO (i) |
ED/e |
Acceptor NBO (j) |
ED/e |
E(2)
kJ/mol |
E(j)-E(i)
a.u. |
F(i,j)
a.u. |
| π -π* |
BD (2) C2 - C3 |
1.795 |
BD*(2) C4 - C5 |
0.309 |
69.58 |
0.28 |
0.06 |
| |
|
|
BD*(2) C9 - N11 |
0.209 |
76.65 |
0.28 |
0.06 |
| σ -σ* |
BD (1) C3 - H8 |
1.972 |
BD*(1) S1 - C2 |
0.028 |
23.39 |
0.73 |
0.06 |
| |
|
|
BD*(1) C2 - C3 |
0.021 |
7.57 |
1.12 |
0.04 |
| |
|
|
BD*(1) C4 - C5 |
0.014 |
8.08 |
1.11 |
0.04 |
| π -π* |
BD (2) C4 - C5 |
1.846 |
BD*(2) C2 - C3 |
0.351 |
66.02 |
0.30 |
0.06 |
| σ -σ* |
BD (1) C4 - H7 |
1.976 |
BD*(1) S1 - C5 |
0.017 |
18.95 |
0.75 |
0.05 |
| |
|
|
BD*(1) C2 - C3 |
0.021 |
8.03 |
1.12 |
0.04 |
| |
|
|
BD*(1) C4 - C5 |
0.014 |
5.73 |
1.12 |
0.04 |
| π -π* |
BD (2) C14 - O15 |
1.980 |
BD*(2) C16 - C17 |
0.335 |
15.10 |
0.40 |
0.04 |
| π -π* |
BD (2) C16 - C17 |
1.633 |
BD*(2) C14 - O15 |
0.277 |
65.98 |
0.3 |
0.06 |
| |
|
|
BD*(2) C18 - C21 |
0.277 |
89.54 |
0.29 |
0.07 |
| |
|
|
BD*(2) N19 - C23 |
0.366 |
69.71 |
0.27 |
0.06 |
| π -π* |
BD (2) C18 - C21 |
1.636 |
BD*(2) C16 - C17 |
0.335 |
74.85 |
0.28 |
0.06 |
| |
|
|
BD*(2) N19 - C23 |
0.366 |
122.13 |
0.27 |
0.08 |
| π -π* |
BD (2) N19 - C23 |
1.706 |
BD*(2) C16 - C17 |
0.335 |
112.97 |
0.32 |
0.08 |
| |
|
|
BD*(2) C18 - C21 |
0.277 |
52.59 |
0.33 |
0.06 |
| n -π* |
LP (2) S1 |
1.622 |
BD*(2) C2 - C3 |
0.351 |
87.15 |
0.27 |
0.07 |
| |
|
|
BD*(2) C4 - C5 |
0.309 |
91.55 |
0.26 |
0.07 |
| n -π* |
LP (2) N12 |
1.667 |
BD*(2) C9 - N11 |
0.209 |
115.14 |
0.29 |
0.08 |
| |
|
|
BD*(2) C14 - O15 |
0.017 |
4.44 |
0.88 |
0.03 |
| |
|
|
BD*(2) C14 - O15 |
0.277 |
190.41 |
0.32 |
0.11 |
| n -σ* |
LP (1) O15 |
1.855 |
BD*(1) N12 - C14 |
0.084 |
118.2 |
0.67 |
0.12 |
| |
|
|
BD*(1) C14 - C16 |
0.069 |
80.37 |
0.66 |
0.10 |
| n -σ* |
LP (1) N19 |
1.916 |
BD*(1) C16 - C17 |
0.033 |
39.33 |
0.90 |
0.08 |
Table 4: The second order perturbation theory analysis of Fock Matrix in NBO basis for T2CNH.
The larger E(2) value shows the intensive interaction between electron donors and electron acceptors. The strong intramolecular hyper conjugative interactions of the σ and π electrons of the C=C, C=N to the anti C=C, C=N bond of the ring as well as C=O group leads to stabilization of some part of the ring system in T2CNH. In the present study, the π-character of the bond plays an important role on comparing with σ bond character. The hyper conjugative interactions π(C2-C3)→π*(C9-N11), π(C4- C5)→π*(C2-C3), π(C16-C17)→π*(C18-C21), π(C18-C21)→π*(N19-C23), π(N19-C23)→π*(C16-C17) and π(C14-O15)→π*(C16-C17) transfer stabilization energy: 76.65, 66.02, 89.54, 122.13, 112.97 and 15.10 KJ/mol to the molecular system. The lone pair of sulphar, nitrogen and oxygen atoms play great role in T2CNH molecule. The S1, N12 & O15 atoms transfer maximum energy 91.55, 190.41 and 118.20 KJ/mol to (C4-C5), (C14-O15) & (N12-C14) bonds, respectively. The maximum hyperconjucative E(2) energy of heteroatoms during the inter-molecular interaction leads the molecule towards medicinal and biological applications [28]. The bond σ(C3-H8) transfer more energy (23.39 KJ/mol) to σ*(S1-C2) bond on comparing with energy transfer (18.95 KJ/mol) from σ(C4-H7) to σ*(S1-C5). Hence the ν(S1-C2) mode observe at higher frequency 791 cm-1 (mode no: 43) than the ν(S1-C5) mode (718 cm-1/mode no: 44).
HOMO-LUMO Analysis
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) analysis is very important parameters for quantum chemistry. The energy values of HOMO (π-donor) and LUMO (π-acceptor) and its energy gap which reflects the chemical activity of the molecule. The HOMO and LUMO energy was calculated by B3LYP/6-311++G(d,p) level of theory. The frontier molecular orbitals (FMOs) of T2CNH are listed in Table 5. The atomic compositions of FMOs are shown in Figure 4. The HOMO is located over the thiophene and hydrozone moieties. The LUMO is located over pyridine ring. The LUMO transition implies that an ED transfer to pyridine ring via hydrazone linkage. The HOMO and LUMO energies are predicted as -6.248 eV and -2.223 eV, respectively. The calculated HOMOLUMO energy gap is 4.025 eV, which explains the eventual charge transfer taking place within the present molecule. The physicochemical properties are also listed in Table 6.
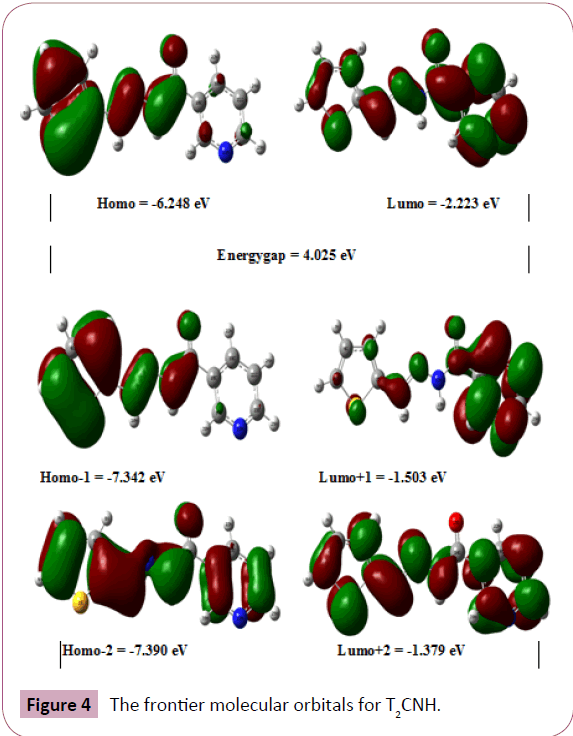
Figure 4: The frontier molecular orbitals for T2CNH.
| Occupancy |
Orbital energies (a.u) |
Orbital energies (eV) |
Kinetic energies (a.u) |
| O56 |
-0.292 |
-7.947 |
1.641 |
| O57 |
-0.284 |
-7.740 |
1.641 |
| O58 |
-0.271 |
-7.390 |
1.582 |
| O59 |
-0.269 |
-7.342 |
2.186 |
| O60 |
-0.229 |
-6.248 |
1.567 |
| V61 |
-0.081 |
-2.223 |
1.590 |
| V62 |
-0.055 |
-1.503 |
1.586 |
| V63 |
-0.055 |
-1.503 |
1.424 |
| V64 |
-0.019 |
-0.519 |
0.488 |
| V65 |
-0.009 |
-0.255 |
0.535 |
Table 5: The frontier molecular orbital of T2CNH.
| Parameters |
Values |
| HOMO |
-6.248 eV |
| LUMO |
-2.223 eV |
| Energy gap |
4.025 eV |
| Ionization potential (IP) |
6.248 eV |
| Electron affinity (EA) |
2.223 eV |
| Electrophilicity Index (ω) |
2.562 |
| Chemical Potential (µ) |
7.359 |
| Electro negativity (χ) |
-7.359 |
| Hardness (η) |
-4.025 |
Table 6: The Physico-Chemical properties of T2CNH.
UV-Vis Spectral Analysis
The UV-Visible absorption spectrum of T2CNH was recorded in the range of 250-350 nm is shown in Figure 5. All the structures allow strong π-π* (or) σ-σ* transition in the UV-Vis region with high extinction coefficients. On the basis of fully optimized ground state structure at TD-DFT/B3LYP/6-311++G(d,p) calculation has been used to determine the low-lying excited states of T2CNH. The calculated results involving the vertical excitation energies, oscillator strength (f) and wavelength are carried out and compared with measured experimental wavelength. Typically, according to Franck-Condon principle, the maximum absorption peaks (λmax) in a UV-Vis spectrum corresponds to vertical excitation. It is evident from the Table 7 that the calculated absorption maxima values have been found to be 332, 301 and 286 nm which correlates well with the experimental values 340 and 290 nm. The more intense band at 340 nm has maximum oscillator strength (f=0.6909), corresponds to Homo-Lumo transition and is mostly characterized as n-π* type. This type of transition is attributed to the presence of large no of free lone pairs of electrons available on sulphar (S1), Nitrogen (N11, N12) and oxygen (O15) atoms. The experimental and theoretical UVVis absorption spectrum is shown in Figure 5. The density of states spectrum of T2CNH is shown in Figure 6. It was used to calculate group contributions to the molecular orbitals (HOMO and LUMO). DOS plot shows population analysis per orbital and demonstrates a simple view of the character of the molecular orbitals (MOs) in a certain energy range.
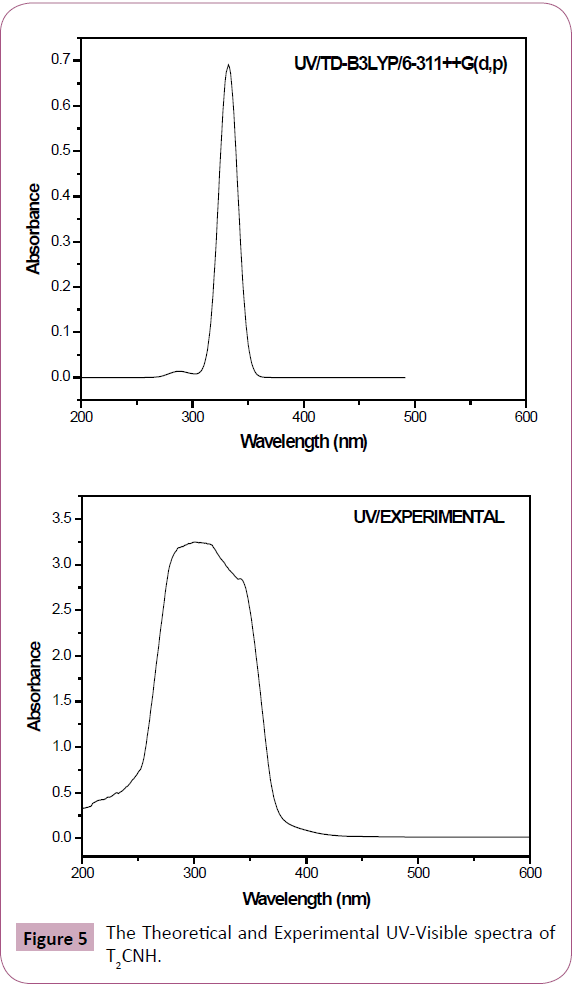
Figure 5: The Theoretical and Experimental UV-Visible spectra of T2CNH.
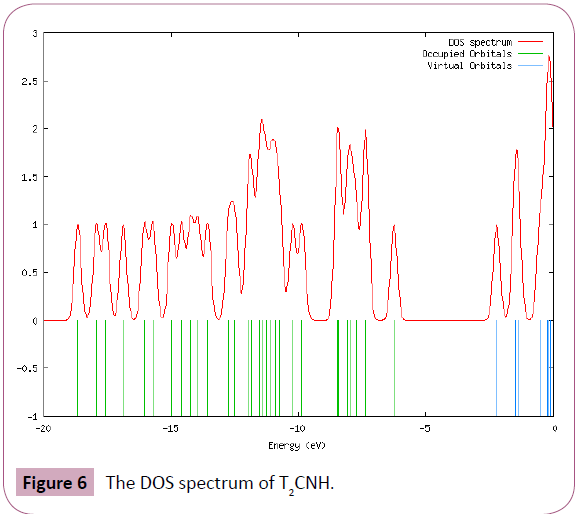
Figure 6: The DOS spectrum of T2CNH.
Calculated at
B3LYP/
6-311++G(d,p) |
Oscillator strength |
Calculated
Band gap (ev/nm) |
Experimental
Band gap (nm) |
Type |
| Excited State 1 |
Singlet-A (f=0.6909) |
3.7311 eV/332.30 nm |
340 |
π-π* |
| 60 -> 61 |
0.6388 |
-4.0254 |
|
|
| 60 -> 62 |
0.1325 |
-4.7451 |
|
|
| Excited State 2 |
Singlet-A (f=0.0043) |
4.1118 eV/301.54 nm |
|
|
| 59 -> 61 |
0.6467 |
-5.1187 |
|
|
| 59 -> 62 |
0.1819 |
-5.8384 |
|
|
| Excited State 3 |
Singlet-A (f=0.0126) |
4.3234 eV/286.77 nm |
290 |
π-π* |
| 60 -> 62 |
0.4175 |
-4.7451 |
|
|
| 60 -> 63 |
0.5580 |
-4.8692 |
|
|
Table 7: The electronic transition of T2CNH.
MEP Analysis
The molecular electrostatic potential (MEP) is widely used as a reactivity map displaying most probable regions for the electrophilic attack of charged part on organic molecule. MEP plot provides a simple way in predicting the interaction of different geometries. In order to predict the reactive sites for electrophilic and nucleophilic attacks of the T2CNH, MEP was calculated with DFT/B3LYP/6-311++G(d,p) level of theory. The negative (red color) and positive (blue color) regions of MEP are related to electrophilic and nucleophilic reactivity respectively is shown in Figure 7. The negative region is located over the carbonyl group and the positive region is located over Hydrogen atom in the hydrazone linkage.
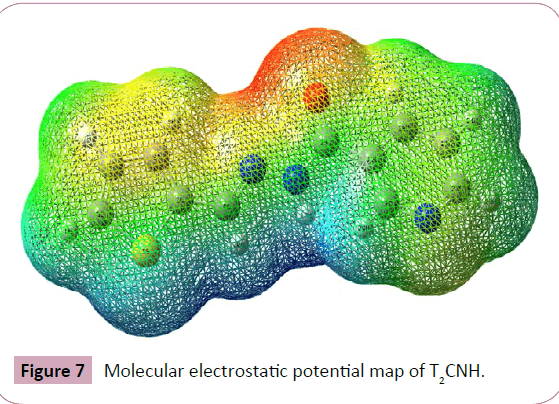
Figure 7: Molecular electrostatic potential map of T2CNH.
Mulliken Charges Analysis
The Mulliken atomic charge calculation has an important role in the application of quantum chemical calculation to molecular system, because the atomic charges should affect dipole moment, polarizability, electronic structure and more a lot of properties of molecular systems. The total atomic charges of T2CNH are calculated by Mulliken population analysis with B3LYP/6-311++G (d,p) basis set and its values are listed in Table 8. The Mulliken atomic charge plot for T2CNH is shown in Figure 8. The C9/C3 atoms have the highest negative/positive charges, respectively among the other atoms in T2CNH due to the resonance.
| Atoms |
Charges |
Atoms |
Charges |
Atoms |
Charges |
| S1 |
-0.6516 |
H10 |
0.1263 |
N19 |
-0.0124 |
| C2 |
0.2759 |
N11 |
0.1794 |
H20 |
0.1758 |
| C3 |
1.9917 |
N12 |
-0.0550 |
C21 |
-0.0964 |
| C4 |
-1.2036 |
H13 |
0.2477 |
H22 |
0.2280 |
| C5 |
0.4451 |
C14 |
-0.7857 |
C23 |
-0.3517 |
| H6 |
0.2687 |
O15 |
-0.2925 |
H24 |
0.1897 |
| H7 |
0.1582 |
C16 |
0.9920 |
H25 |
0.1903 |
| H8 |
0.2452 |
C17 |
-0.5899 |
|
| C9 |
-1.7721 |
C18 |
0.0967 |
Table 8: The Mulliken atomic charges of T2CNH.
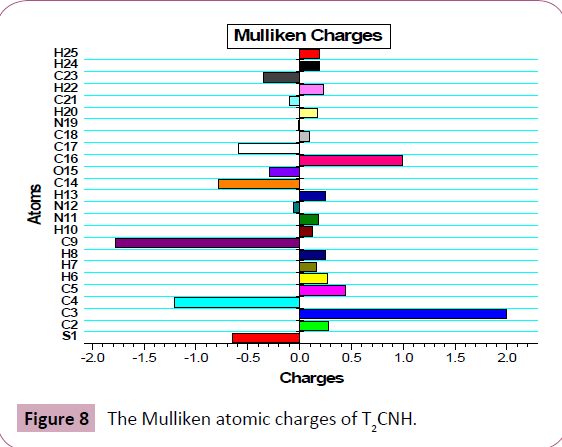
Figure 8: The Mulliken atomic charges of T2CNH.
`
Thermodynamical Properties
Several thermodynamic parameters have been calculated using DFT/B3LYP/ 6-311++G(d,p) basis set are listed in Table 9. The thermodynamic parameters viz, entropy (S0m), enthalpy (ΔH0m) and heat capacity at constant pressure (C0 p,m) for T2CNH was calculated from the theoretical harmonic wavenumbers obtained from B3LYP/6-311++G(d,p) basis set in the range 100-1000 k and listed in Table 10. It is evident from the Table 10 that the thermodynamic parameters increase with rise of temperature due to the fact that the molecular vibrational intensities increase with temperature. The correlation equations between these thermodynamic parameters and temperature were fitted by parabolic formula and the regression coefficient is also given in the parabolic equation. The correlation relation between the thermodynamic parameters and temperature are as follows:
| Parameters |
B3LYP/6-311++G(d,p) |
| Total Energies |
-1062.41677 |
| Zero-point Energy |
114.99338 (Kcal/Mol) |
| Rotational constants (GHZ) |
1.78813 |
| |
0.15572 |
| |
0.14465 |
| Entropy |
|
| Total |
122.336 |
| Translational |
42.214 |
| Rotational |
33.345 |
| Vibrational |
46.777 |
Table 9: The calculated total energy (a.u), zero point vibrational energies (kcal/mol), rotational constants (GHz) and entropy (cal/mol K-1) for T2CNH.
| T (K) |
S (Jmol-1K-1) |
Cp (Jmol-1K-1) |
ddH (kJmol-1) |
| 100 |
349.07 |
100 |
6.95 |
| 200 |
436.15 |
159.71 |
19.86 |
| 298.15 |
511.96 |
224.53 |
38.7 |
| 300 |
513.36 |
225.74 |
39.12 |
| 400 |
586.95 |
287.34 |
64.85 |
| 500 |
656.77 |
338.54 |
96.24 |
| 600 |
722.24 |
379.34 |
132.21 |
| 700 |
783.25 |
411.8 |
171.83 |
| 800 |
840.01 |
438.01 |
214.36 |
| 900 |
892.88 |
459.52 |
259.27 |
| 1000 |
942.25 |
477.43 |
306.15 |
Table 10: Thermodynamic properties of T2CNH at different temperatures.
C0p,m= 4.44266+0.01876T+1.6571 × 10-5 T2 (R2=0.99929)
S0 m= 1.35549+0.00572T+5.0560 × 10-5 T2 (R2=0.99997)
ΔH0m= 3.15466+0.01332T+1.1767 × 10-5 T2 (R2=0.99944)
The correlation graphs between various thermodynamic functions and temperature are graphically presented in Figure 9.
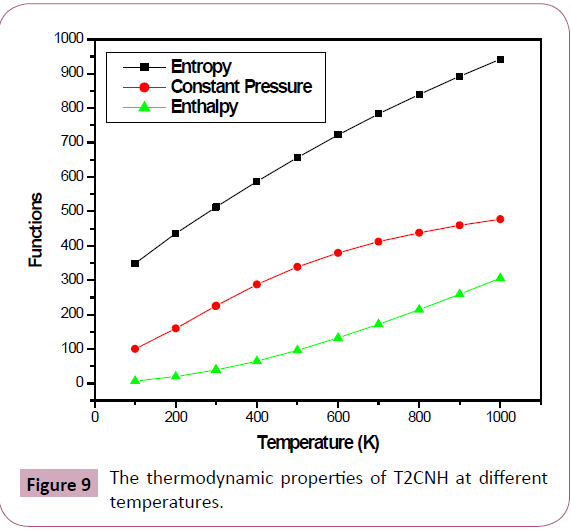
Figure 9: The thermodynamic properties of T2CNH at different temperatures.
Conclusion
The complete vibrational analysis has been performed to using quantum chemical calculation at DFT method for the first time to the title molecule T2CNH. The calculated bond parameters are good agreement with the related single crystal X-ray diffraction (XRD) data. In T2CNH, the thiophene and hydrazone moieties are co-planar, which shows a good conjuction between p-orbitals of all atoms of thiophene and hydrazone moieties. The vibrational data of T2CNH are well supported by the harmonic and related literature values. The β0 value of T2CNH molecule is found to be 1.5861 × 10-30 esu, which is four time greater thatn that of urea. The NBO result reflects the charge transfer with in the molecule and the maximum energy takes place during π-π* transition. The Homo-Lumo band gap was calculated about 4.025 eV, which leads the T2CNH molecule to become less stable and more reactive. The recorded UV-Vis spectral values are agree well with calculated values. MEP surface predict the reactive sites for electrophilic and nucleophilic attack. In addition, Mulliken atomic charges, zero point energy and thermodynamic properties are also calculated.
References
- The Encyclopœdia Britannica (1882), Nature 25: 313-315.
- Molvi KI, Vasu KK, Yerande SG, Sudarsanam V, Haque N (2007) Syntheses of new tetrasubstituted thiophenes as novel anti-inflammatory agents. Euro J Medi Chem 42: 1049-1058.
- Rai NS, Kalluraya B, Lingappa B, Shenoy S, Puranic VG (2008) Convenient access to 1,3,4-trisubstituted pyrazoles carrying 5-nitrothiophene moiety via 1,3-dipolar cycloaddition of sydnones with acetylenic ketones and their antimicrobial evaluation. Euro J Medi Chem 43: 1751-1720.
- Ashalatha BV, Narayana B, Raj KKV, Kumari NS (2008) Synthesis of some new bioactive 3-amino-2-mercapto-5,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidin-4(3H)-one derivatives. Euro J Medi Chem 42: 719-728.
- Polivka Z, Holubek J, Svatek E, Metys J, Protiva M (1984) Potential hypnotics and anxiolytics: Synthesis of 2-bromo-4-(2-chlorophenyl)-9-[4-(2-methoxyethyl)piperazino]-6H-thieno[3,2,4-triazolo[4,3-a]-1,4-diazepine and of some related compounds. Collect Czech Chem Commun 49: 621-623.
- Noguchi H, Kitazumi K, Mori M, Shiba T (2004) Electroencephalographic Properties of Zaleplon, a Non-Benzodiazepine Sedative/Hypnotic, in Rats. J Pharm Sci 94: 246-251.
- Kim S, Yoon JY (2004) Hydrazones in science and synthesis. Sci Synth 27: 671-722.
- Brehme R, Enders D, Fernandez R, Lassaletta JM, Aldehyde N (2007) N-Dialkylhydrazones as Neutral Acyl Anion Equivalents: Umpolung of the Imine Reactivity. Eur J Org Chem 34: 5629-5660.
- Belskaya NP, Dehaen W, Bakulev VA (2010) Synthesis and properties of hydrazones bearing amide, thioamide and amidine functions. Archive Org Chem 275-332.
- Bhole RP, Bhusari KP (2009) Synthesis, antimycobacterial activity and 3-d qsar studies of some new derivatives of p-hydroxybenzohydrazide. QSAR Comb Sci 28: 1405-1417.
- Loncle C, Brunel JM, Vidal N, Dherbomez M, Letourneux Y (2004) Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J MedChem 39: 1067-1071.
- KK Bedia, Elcin O, Seda U, Fatma K, Nathaly S (2006) Synthesis and characterization of novel hydrazide–hydrazones and the study of their structure–antituberculosis activity. Eur J Med Chem 41: 1253-1261.
- Subashchandrabose S, Meganathan C, Erdogdu Y, Saleem H, Jayakumar C (2013) Vibrational and conformational analysis on N1-N2-bis(pyridine-4-yl)methylene)benzene-1, 2-diamine. J Mol Struct 1042: 37-44.
- Subramaninan N, Sundaraganesan N, Jayabharathi JA (2010) Molecular structure, spectroscopic (FT-IR, FT-Raman, NMR, UV) studies and first order molecular hyperpolarizabilities of 1,2-bis (3-methoxy-4-hydroxy benzlidene) hydrazine by density functional method. Spectrochim Acta A Mol Biomol Spectrosc 76: 259-269.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, et al. (2004)Theoretical and Computational Aspects of Magnetic Organic Molecules. Gaussian Inc, Wallingford, CT.
- Schlegel HB(1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3: 214-218.
- Jamróz MH (2006)Vibrational modes of 2,6-, 2,7-, and 2,3-diisopropylnaphthalene, A DFT study.J Mol Struct 787: 172-183.
- Michalska D(2003) Raint Program, Wroclaw University of Technology, Poland.
- Michalska D, Wysokinski R(2005)The prediction of Raman spectra of platinum(II) anticancer drugs by density functional theory.Chem Phys Lett 403: 211-217.
- Fleming GD, Koch R, Vallete MMC (2006) Theoretical study of the syn and anti thiophene-2-aldehyde conformers using density functional theory and normal coordinate analysis. Spectrochim Acta AMol Biomol Spectrosc65: 935-945.
- Balachandran V, Janaki A, Nataraj A (2014) Theoretical investigations on molecular structure, vibrational spectra, HOMO, LUMO, NBO analysis and hyperpolarizability calculations of thiophene-2-carbohydrazide. Spectrochim Acta A Mol Biomol Spectrosc118: 321-330.
- Unal A, Eren B (2013) FT-IR, dispersive Raman, NMR, DFT and antimicrobial activity studies on 2-(Thiophen-2-yl)-1H-benzo[d]imidazole. Spectrochim Acta AMol Biomol Spectrosc 114: 129-136.
- Brathen O, Kveseth K, Nielsen CJ, Hagen K (1986) Molecular structure and conformational equilibrium of gaseous thiophene-2-aldehyde as studied by electron diffraction and microwave, infrared, Raman and matrix isolation spectroscopy. J Mol Struct 145: 45-68.
- Geiger DK, Geiger HC, Williams L, Noll BC (2012) 2-(Thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1 H –benzimidazole. Acta Cryst E: Structure Reports Online 68: o420.
- Rauhut G, Pulay P (1995) Transferable Scaling Factors for Density Functional Derived Vibrational Force Fields. J Phys Chem 99: 3093-3100.
- Bellamy LJ (1980) The infrared spectra of complex molecules, vol 2 champman and Hall, London.
- Babu NR, Subashchandrabose S, Padusha MSA, Saleem H, Manivannan V, et al. (2014) Synthesis and structural characterization of (E)-N′-((Pyridin-2-yl) methylene) benzohydrazide by X-ray diffraction, FT-IR, FT-Raman and DFT methods. J Mol Struct 1072: 84-93.
- Varsanyi G (1974) Assignments of vibrational spectra of 700 Benzene Derivatives, Wiley, New York.
- Sathyanarayanan DN (2000) Vibrational spectroscopy theory and Applications, 446-447.
- Varsanyi G (1973) Assignments for Vibrational Spectra of Seven Hundred Benzene derivatives, 1/2 Academic Kiaclo.
- Jag M (2001) Organic Spectroscopy Principles and Application, (2nd edn), Narosa Publishing house, New Delhi.
- Lorenc J (2012) Dimeric structure and hydrogen bonds in 2-N-ethylamino-5-metyl-4-nitro-pyridine studied by XRD, IR and Raman methods and DFT calculations. Vib Spectrosc 61: 112-123.
- Krishnakumar V, Dheivamalar S, Xavier RJ, Balachandran V (2006) Analysis of vibrational spectra of 4-amino-2, 6-dichloropyridine and 2-chloro-3, 5-dinitropyridine based on density functional theory calculations. Spectrochim Acta A Mol Biomol Spectrosc65: 147-154.
- Sundaraganesan N, Saleem H, Mohan S (2003) Vibrational spectra, assignments and normal coordinate analysis of 2-amino-5-bromopyridine.Spectrochim Acta A Mol Biomol Spectrosc59: 1113-1118.
- Silverstein M, Basseller CG, Morill C (1981) Spectrometric identification of organic compound, Wiley; Network.
- Badawi HM (2009) Structural stability, C–N internal rotations and vibrational spectral analysis of non-planar phenylurea and phenylthiourea. Spectrochim Acta A Mol Biomol Spectrosc72: 523-527.
- James C, Ravikumar C, Sundius T, Krishnakumar V, Kesavamoorthy R, et al. (2008) FT-Raman and FTIR spectra, normal coordinate analysis and ab initio computations of (2-methylphenoxy) acetic acid dimer.Vib Spectrosc 47: 10-20.
- Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge University Press.
- Ott JB, Boerio-Goates J (2000) Calculations from Statistical Thermodynamics.