Research Article - (2017) Volume 3, Issue 2
Raed A Al-khayyal1, Fawaz A Al-Mousa1 and Afaf M Attia2 and Ahmed R Ragab1,2*
1General Directorate of Poisons Control Centers- Ministry of Health, Riyadh, Saudi Arabia
2Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Corresponding Author:
Ahmed R Ragab
Department of Forensic Medicine and Clinical Toxicology
Faculty of Medicine, Mansoura University
Mansoura, Egypt
Tel: 050-344725
E-mail: ahmedrefat1973@yahoo.com
Received date: August 26, 2017; Accepted date: August 29, 2017; Published date: August 31, 2017
Citation: Ragab AR, A Al-khayyal R, Al-Mousa FA, Attia Am (2017) Efficiency Evaluation of Urine Collection Vessels with Impeded Urine Adulteration/Substance of Abuse (SOA) Rapid Detection Test Strips. J Drug Abuse. Vol. 3 No. 2:11
Laboratory testing for drugs of abuse has become standard practice in many settings both forensic and clinical. Sending the specimen to any laboratory is waste of time and sometimes the rapid urine testing is a target. A limitation inherent in all urine drug testing is the possibility of sample adulteration or substitution. The aim of the current study is to prove the validity by deeply investigated procedures for urine collection vessels with impeded urine adulteration/substance of abuse (SOA) Test Strips (two types of investigated kits) and evaluation of their total turnaround time and efficacy for cannabinoids, opiates, benzodiazepines, tramadol and amphetamines in-comparison with immunoassay screening and GC-MS confirmatory procedures. Both Investigated Kits have the capability of detecting variable procedures of adulteration by a high efficiency degree. Therefore, SOA tests with rapid releasing results can improve patient management protocol with few percentage of pseudo-positivity/negativity results, unfortunately the operators immediate start the clinical action and realize the limitations of the test and wait for follow-up confirmation testing as appropriate before definitive medico-legal action.
Keywords
Rapid detection kit; Substance of abuse; Urine adulteration
Introduction
Laboratory testing for drugs of abuse has become standard practice in many settings both forensic and clinical. Urine is the predominant specimen, but other specimens are possible including hair, nails, sweat and oral fluid. Point-of-care test kits provide for rapid analysis at the site where specimens are collected allowing for immediate action on the results [1]. Urine drug testing is used in global areas to test drug use and monitor treatment programs. Sending the specimen to any laboratory is waste of time and sometimes the rapid urine testing is a target [2].
Urine is the predominant specimen for drug analysis, although other specimens are possible: oral fluid, blood, hair, sweat, and meconium. Urine is easy to collect, relatively noninvasive, can be collected with sufficient volume for confirmatory and repeat testing (if required) and contains drug and metabolites in high concentrations compared to other body fluids [3]. Urine has little protein and other contaminants, so is a relatively clean sample for chromatography and MS processing compared to hair or blood. Urine reflects drug exposure over the prior several days and detection will depend on the metabolism and timing of ingestion and sample collection. Urine is also subject to hydration status of the patient, so drug concentrations in urine do not correlate with the clinical signs and symptoms [4].
Drug testing occurs in two phases: screening and confirmation. Historically, the initial screen has been a chemical test, thin layer chromatography or immunoassay (IA) that can provide a reasonable turnaround time (under an hour for stat specimens) with minimal labor and resources. Today, most screening tests are homogeneous IAs that can be automated on the core laboratory chemistry analyzers. A single specimen aliquot can be utilized for electrolytes, metabolites (such as glucose or creatinine) and drugs all on the same instrument, enhancing efficiency and reducing the volume of sample required from the patient. These IAs produce results in less than 10 min and results can be auto verified to the patient’s electronic medical record minimizing labor at every step of testing, from processing to analysis to reporting of results [1].
A limitation inherent in all urine drug testing is the possibility of sample adulteration or substitution. The simplest mechanism to prevent adulteration is to have the operator observe collection, but this is not always possible or desirable due to privacy concerns. Some urine test kits contain adulterant detection for a variety of common additives and come with thermometers on the test cup. These tools are useful in detecting several common adulterants and will detect substitution with a room temperature sample if read promptly; however, they will not detect substitution with pre-warmed urine [5,6].
Aim of the Work
The aim of the current study is to prove the validity by deeply investigated procedures for urine collection vessels with impeded urine Adulteration/SOA Test Strips (two types of Investigated Kits), and evaluation of their total turnaround time and detection efficacy for cannabinoids, opiates, benzodiazepines, tramadol and amphetamines in-comparison with immunoassay, GC-MS and LC-MS.
Materials
This study was conducted on 200 suspected substance abuse cases that referring to Riyadh, Makah and Dammam poison control centers. 200 investigated kits “100 in each cup Urine Collection Vessel/ Strip –Adulteration /SOA” were tested.
Kits
1) Type I investigated kits "Abon Biopharm Multi-Drug Screen"
The examiners provided urine directly into the investigated vessels and closed it with the white cap. Then after its reading, the investigated urine sample was spelled in empty clean container and tested by strip Adulterant/DOA kits. The investigator validated the temperature sensitivity immediately and then read out the possibility of adulteration and substance of abuse within the recommended time (3-5 min).
2) Type II investigated kits “Sure Screen Diagnostics”
The examiners provided urine directly into the routine empty clean container. Then after its reading, the investigated urine sample was spelled in empty clean container and tested by strip Adulterant/DOA kits. The investigator validated the temperature sensitivity immediately, and then read out the possibility of adulteration and substance of abuse within the recommended time (3-5 min).
3) Immunoassay kits
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Opiate Assay:
For qualitative analysis of opiates in human urine.
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Benzodiazepine Assay:
For qualitative analysis of benzodiazepines in human urine.
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Cannabis Assay:
mFor qualitative analysis of cannabis in human urine.
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Amphetamines Assay:
For qualitative analysis of amphetamines in human urine.
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Barbiturates Assay:
For qualitative analysis of barbiturates in human urine.
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Tramadol Assay:
For qualitative analysis of tramaol in human urine.
- ARCHITECT® d.a.u. (drug of abuse in urine) ci 4100 Cocaine Assay:
For qualitative analysis of cocaine in human urine.
Instruments
o ARCHITECT Version ci 4100.
o GC-MS (Hewlett Packard, 6890/5972 )
o LC-MS Thermo Finnegan LTQ FT Ultra High Performance Mass Spectrometer
Methods
Urine sampling
After having informed consent, forty ml urine was obtained from each patient at time of admission and prior to giving any treatment. Each sample was collected in a dry, labeled container (serial number, age, gender of the patient, clinical provisional diagnosis and date of taking the sample).
Toxicological analysis
Preliminary drug screen test 1 was performed by "Abon Biopharm Multi-Drug Screen" and “Sure Screen Diagnostics”, then by ARCHITECT immunoassay analyzing system. Each urine sample was screened for cannabinoids, opiates, benzodiazepines, tramadol and amphetamines. Confirmatory testing of positive results with quantitative values was performed by Gas Chromatography Mass, Spectrometry (GC-MS) and Liquid Chromatography Mass spectrometry (LC-MS). Cannabinoids, Opiates, Benzodiazepines, Tramadol, Amphetamine, Barbiturates and Cocaine were analyzed with GC–MS after liquid/liquid extraction before GC– MS detection [8]. Cannabis was confirmed by analyzing the presence of tetrahydrocannabinol (THC) using a method based on solid-phase extraction followed by liquid chromatographymass- spectrometry [9].
Drug cut-off levels
Sensitivity of the method is the ability to detect a drug when it exists at or above cut-off levels which are listed in Table 1.
| Drugs of Abuse Type |
Cut-off Level (ng/ml) | |||
|---|---|---|---|---|
| Screening Procedures | Confirmatory Procedure | |||
| Type I Investigated Kits |
Type II Investigated Kits | ARCHITECT Immunoassay Kits |
GC-MS/ LC-MS | |
| Opiates | 300 ng/ml | 300 ng/ml | 300 ng/ml | 200 ng/ml |
| Amphetamines | 300 ng/ml | 300 ng/ml | 300 ng/ml | 250 ng/ml |
| Benzodiazepines | 200 ng/ml | 200 ng/ml | 200 ng/ml | 100 ng/ml |
| Cannabinoids | 25 ng/ml | 25 ng/ml | 25 ng/ml | 15 ng/ml |
| Cocaine | 300 ng/ml | 300 ng/ml | 300 ng/ml | 150 ng/ml |
| Tramadol | 300 ng/ml | 300 ng/ml | 300 ng/ml | 100 ng/ml |
| Barbiturates | 200 ng/ml | 200/ml | 200/ml | 200 ng/ml |
Table 1: Cut-off levels in ng/ml for drugs of abuse tested in urine samples in this study.
Screening methods generally use higher detection cut-offs than do testing methods to reduce the incidence of false negative results [6].
Validity tests
In the present validity procedure; the external appearance, the malleability of usage, the integrity of sealing, the sensitivity of parameters and finally the efficiency of usage were tested. The examined container/strip tested for:
1st both natural well reported adulterated urine samples and artificial adulterated urine samples.
2nd real confirmed positive urine samples by preliminary immunoassay procedures and confirmatory GC-MS procedures.
• The artificial adulteration consists of three degree; mild, moderate and high degree of the adulterant material contaminations. The substance of abuse detection was confirmed with variable diluted concentration of adulterated substances, double, triple, quadriple, …, 10 times, 20 times, 30 times, 40 times, 50 times dilution/ fraction of cutoff point.
• The next mentioned materials were used in the validity proven procedure; Cholox, Vinegar, Detergent, Salt, Straw berry, Flash, Oven cleaner, Lemon Juice, Soda, Eye drops, Tape water, Deionized water and Warm water.
• The positive substance of abuse samples were investigated after screening immunoassay procedures & confirmatory GC/MS techniques for Amphetamines, Cannabis, Opiates and Benzodiazepines.
• Test categories and result types
Creatinine: Normal- Abnormal “Low/High”
Specific Gravity: Normal -Abnormal “Low/High”
pH: Normal - Abnormal “Low/High”
Nitrite: Normal-Abnormal “High”
Glutaraldehydes: Normal-Abnormal “High”
Oxidants/PCC: Normal Abnormal “Low/High”
Results
This study was conducted on 200 cases of suspected substance of abuse from three poison control centers at Saudi Arabia (Riyadh, Dammam and Makah). As shown in Table 2, the best result appeared within 5 min in type I investigated kits while, it appeared within 4-5 min in type II investigated kits, with clear line landmark and identity for both. In low level of amphetamines, positivity can be detected up to 374.5 ng/ml in type I investigated kits and 325.2 ng/ml in type II investigated kits while, THCs were detected up to 33.6 ng/ml and 35.7 ng/ml in Type I and II investigated kits, respectively “But with weak positivity with a very low level”.
| Type (I) Investigated Kits "Abon Bio pharm Multi-drug screen" |
Type (II) Investigated Kits "Sure Screen Diagnostics" |
|
|---|---|---|
| Time of results appearance | 5 min | 4-5 min |
| Low level of amphetamines | Detected up to 374.5 ng/ml | detected up to 325.2 ng/ml |
| THCs | Detected up to 33.6 ng/ml | Detected up to 35.7 ng/ml |
| Water dilutional effect (as adulterant agent) in low amphetamines/cannabis urinary levels | Persistent positivity up to 2-3 dilutional times | Persistent positivity up to 2 dilutional times |
| Water dilutional effect (as adulterant agent) in high amphetamines/cannabis urinary levels | Persistent positivity up to 30 times | Persistent positivity up to 60 times |
Table 2: Comparison between Type I and II investigated kits regarding time of appearance of the results, detection capability of low amphetamines/ THCs levels and sensitivity of detection for both amphetamines and cannabis in different water dilutions.
As regards the sensitivity of detection for both low level of amphetamines and cannabis in different water dilutions, there is persistence positivity up to 2-3 dilution times and Persistent positivity up to 2 dilution times when using type I investigated kits and type II investigated kits respectively. While, the sensitivity of detection for both high level of amphetamines and cannabis in different water dilutions were persistent positivity up to 30 times and persistent positivity up to 60 times when using type I investigated kits and type II investigated kits respectively.
In Table 3, there were 3 cases showed pseudo amphetamines negative results (negative by investigated kits while positive by preliminary and confirmatory procedures) when using type I investigated kits and 4 cases when using type II investigated kits.
| Type (I) Investigated Kits "Abon Biopharm Multi-drug screen" |
Type (II) Investigated Kits "Sure Screen Diagnostics" |
|
|---|---|---|
| Pseudo-amphetamines NEGATIVE results Negative results by Investigated kits Positive results by preliminary and confirmatory procedures |
Three cases From 81 investigated Amphetamines positive cases at a very low absorbance level “300-400 ng/ml” |
Four cases From 49 investigated Amphetamines positive cases at a very low absorbance level “300-400 ng/ml” |
| Pseudo-benzodiazepines POSITIVE results Positive results by Investigated kits Negative results by preliminary and confirmatory procedures |
One case | |
| Pseudo-benzodiazepines POSITIVE results Positive results by Investigated kits Negative results for benzodiazepines and positive results for opiates by preliminary and confirmatory procedures |
One case | |
| Pseudo-barbiturates POSITIVE results Positive results by Investigated kits Negative results for barbiturates and positive results for benzodiazepines by preliminary and confirmatory procedures |
One case |
Table 3: The false results in Type I and Type II investigated kits.
There was no cases showed pseudo benzodiazepines positive results when using type I investigated kits while, regarding type II investigated kits, there was one case showed pseudo benzodiazepines positive results (positive by investigated kits while negative by preliminary and confirmatory procedures) and one case showed pseudo benzodiazepines positive results (positive results by Investigated kits benzodiazepines while it was positive for opiates by preliminary and confirmatory procedures) and one case showed pseudo benzodiazepines negative results (negative results by Investigated kits for benzodiazepines and positive for barbiturates while, it was positive for benzodiazepines by preliminary and confirmatory procedures)
In Tables 4 and 5, the validity tests were accepted for both types I and II investigated kits as regards sealing, container material, white cup tap, creatinine parameter, nitrite parameter, glutaraldehyde, pH, specific gravity and oxidant/pcc.
| Parameter | Validity status | Comment(s) |
|---|---|---|
| The validity tests results | ||
| Sealing | Accepted | Need sharp object to cut |
| Container material | Accepted | |
| White Cup tap | Accepted | Firmly closed |
| Creatinine parameter | Accepted. | Changeable with different dilution degree |
| Nitrite parameter | Accepted. | |
| Glutaraldehyde | Accepted. | |
| pH | Accepted. | Sensitive with variable degree of normal. Low and high pH |
| Specific Gravity | Accepted. | Changeable with variable dilutions & substitute additional concentrations |
| Oxidant/PCC | Accepted. | In a very high concentration; a gray blue discolorations was given instead of dark blue discolorations |
| Temperature Strip | Accepted | |
| Drug Of Abuse Test Results | ||
| Amphetamines | Accepted | 99.75% Accuracy |
| Tertahydrocannabinol | Accepted | 100% Accuracy |
| Opiates | Accepted | 100% Accuracy |
| Benzodiazepines | Accepted | 100% Accuracy |
| Tramadol | Accepted | 100% Accuracy |
Table 4: The validity tests & drug of abuse test results for type I investigated kits.
| Parameter | Validity status | Comment(s) |
|---|---|---|
| The validity tests results | ||
| Sealing | Accepted | |
| Container material | Accepted | |
| Creatinine parameter | Accepted. | Changeable with different dilution degree. |
| Nitrite parameter | Accepted. | |
| Glutaraldehyde | Accepted. | |
| pH | Accepted. | Sensitive with the degree of pH (Normal, Low and high) |
| Specific Gravity | Accepted. | Changeable with variable dilutions & substitute additional concentrations. |
| Oxidant/PCC | Accepted. | In a very high concentration; purple blue discolorations were given instead of dark blue discolorations. |
| Temperature Strip | Accepted | |
| Drug of Abuse Test Results | ||
| Amphetamines | Accepted | 99.25% Accuracy |
| Tertahydrocannabinol | Accepted | 100% Accuracy |
| Opiates | Accepted | 100% Accuracy |
| Benzodiazepines | Accepted | 94.6% Accuracy |
| Tramadol | Accepted | Need to testify |
Table 5: The validity tests & drug of abuse test results for type II investigated kits.
Drugs of abuse accepted with accuracy 100% for tetrahydricanabinol, opiate, benzodiazepines, tramadol and with accuracy 99.75% for amphetamines using type I investigated kits while, they accepted for type II investigated kits with accuracy 100% for tetrahydrocanabinol and opiate, 94.6% for benzodiazepines and need testify for tramadol and 99.25 % for amphetamines (Figures 1-4).

Figure 1: Type (I) & (2) investigated Kits with: (A) Positive cannabis and amphetamines, (B) Positive tramadol and benzodiazepines and (C) Positive cannabis and opiates.
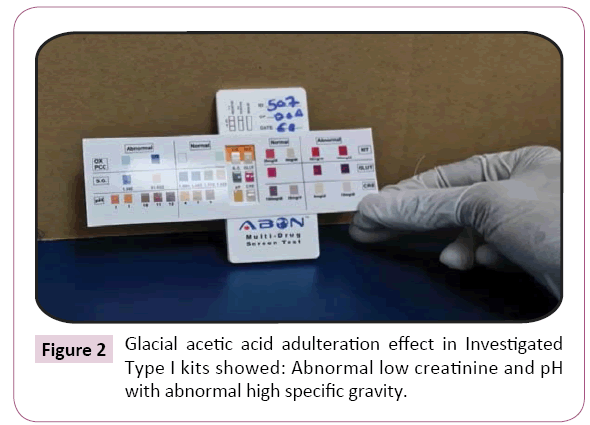
Figure 2: Glacial acetic acid adulteration effect in Investigated Type I kits showed: Abnormal low creatinine and pH with abnormal high specific gravity.
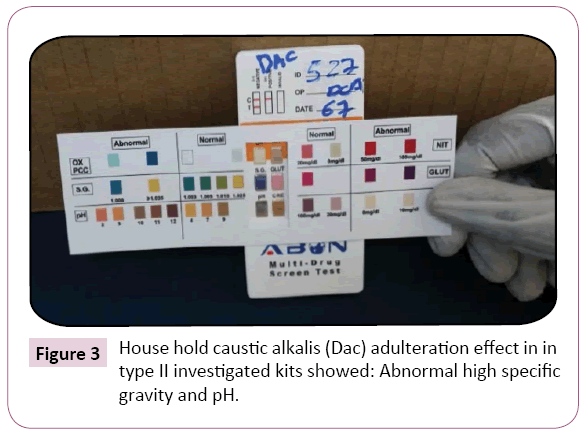
Figure 3: House hold caustic alkalis (Dac) adulteration effect in in type II investigated kits showed: Abnormal high specific gravity and pH.
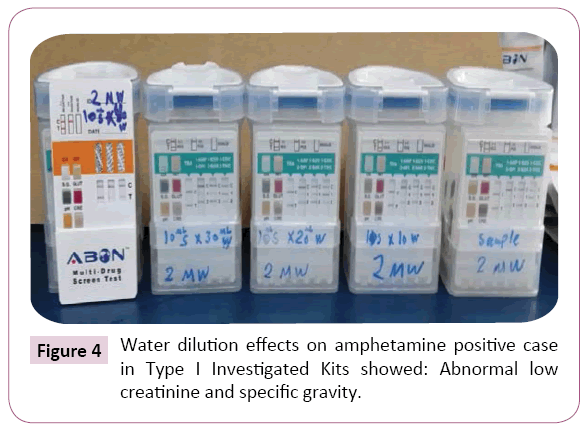
Figure 4: Water dilution effects on amphetamine positive case in Type I Investigated Kits showed: Abnormal low creatinine and specific gravity.
Discussion
Drug testing is an important component of clinical care, occupational health, legal prosecution, rehabilitation, and risk management. The need for drug testing can occur in the emergency room, drug testing can assist in the triage of obtunded patients and those with altered mental status, pre-employment screening, post-accident investigation, and the military [1].
Testing of the drugs in urine is used widely to monitor drug treatment compliance and to detect drug used. Transportation of the samples to laboratories is time- consuming and sometimes the conditions considered critical as regards time [2].
So, the aim of the current study is to prove the validity by deeply investigated procedures for urine collection vessels with impeded urine Adulteration/SOA Test Strips (Type I Investigated Kits "Abon Biopharm Multi-Drug Screen and Type II Investigated Kits “Sure Screen Diagnostics), and evaluation of their total turnaround time and efficacy for cannabinoids, opiates, benzodiazepines, tramadol and amphetamines in-comparison with immunoassay and GC-mass.
The current study revealed that the percentages of positive urine samples using ARCHITECT immunoassay analyzing system were (30.2%, 15.77%, 12.45%, 7.6% and 24%) for cannabinoids, opiates, benzodiazepines, amphetamines and tramadol respectively.
Positive results obtained by ARCHITECT immunoassay analyzing system were confirmed by GC/MS and LC-MS which revealed that the prevalence rates were as follows: cannabinoids (17.25%), opiates (7.25%), benzodiazepines (11.33%), amphetamines (8.67%) and tramadol (21%).
Immunoassays are routinely used for analysis of a large number of analytes tested in clinical laboratories, and these assays use photometric, luminometric or fluorometric signals and homogeneous or heterogeneous formats. Serum and plasma is the main specimen types used for immunoassays, although alternate types of specimens have also been used. Although immunoassays exhibit excellent sensitivity and specificity, a major limitation of immunoassays is interference from various endogenous (heterophilic antibody, rheumatoid factors, high bilirubin, etc.) and exogenous factors (drug metabolites and structurally similar compounds) that may falsely elevate or reduce the true value of the analyte [10].
The results of current study revealed that the best result appeared within 5 min in type I investigated kits while, it appeared within 4-5 min in type II investigated kits, with clear line landmark and identity for both. In low level of amphetamines, positivity can be detected up to 374.5 ng/ml, in type I investigated kits and 325.2 ng/ml, in type II investigated kits While, THCs were detected up to 33.6 ng/ml and 35.7 ng/ml, in Type I and II investigated kits respectively “But with weak positivity with a very low level”.
Wiencek et al. [1] stated that most screening tests are homogeneous IAs that can be automated on the core laboratory chemistry analyzers. A single specimen aliquot can be utilized for electrolytes, metabolites (such as glucose or creatinine) and drugs all on the same instrument, enhancing efficiency and reducing the volume of sample required from the patient. These IAs produce results in less than 10 min and results can be autoverified to the patient’s electronic medical record minimizing labor at every step of testing, from processing to analysis to reporting of results.
As regards the sensitivity of detection for both low level of amphetamines and cannabis in different water dilutions, the current study showed persistence positivity up to 2-3 dilution times and persistent positivity up to 2 dilution times when using type I investigated kits and type II investigated kits respectively. While, the sensitivity of detection for both high level of amphetamines and cannabis in different water dilutions were persistent positivity up to 30 times and persistent positivity up to 60 times when using type I investigated kits and type II investigated kits, respectively.
Accordingly, and similar to central laboratory tests, validations of moderately complex tests performed at the point of care must document several performance characteristics before patient results are reported. These characteristics include precision, accuracy, analytical measurement range and the detection limit or cutoff [11].
On site or Point-of-care test kits offers fast turnaround of test results which has the potential for improving patient care and efficiency of management decisions. Rapid drug testing in an urban emergency room significantly reduced turnaround time of results by 69% an improvement that also decreased overall length-ofstay [12].
Holm-Hansen et al. [13] stated that a limitation inherent in all urine drug testing is the possibility of sample adulteration or substitution. The simplest mechanism to prevent adulteration is to have the operator observe collection, but this is not always possible or desirable due to privacy concerns. Some urine test kits contain adulterant detection for a variety of common additives and come with thermometers on the test cup. These tools are useful in detecting several common adulterants and will detect substitution with a room temperature sample if read promptly; however, they will not detect substitution with prewarmed urine.
In the present study, there were 3 cases showed pseudo amphetamines negative results (negative by investigated kits while positive by preliminary and confirmatory procedures) when using type I investigated kits and 4 cases when using type II investigated kits.
Moody et al. [14] observed that the false positive arose from detection of sympathomimetic amines such as pseudoephedrine. In the current study, both methods showed pseudo false negative results and this may be due to their cross reactivity for methamphetamines.
Another study of 400 arrestee urine samples demonstrated that the false-positive rates for the amphetamine screening test (2.12%-3.75%) dropped when accounting for presence of MDMA (<2%) and opioid false-positive rates dropped when considering the presence of hydrocodone/hydromorphone after confirmation (<0.3%); in both cases.
Cross-reactivity was the underlying cause for false positivity. This study also found that the overall error rates of rapid testing conducted by trained medical technologists (0.8%) were significantly lower than the error rates of police officers (2.5%); highlighting the dependence of POCT performance on operator training and suggesting that additional training is needed before routine use of rapid drug testing by officers [15].
In the current work, there was no cases showed pseudo benzodiazepines positive results when using type I investigated kits while, regarding type II investigated kits, there was one case showed pseudo benzodiazepines positive results (positive by investigated kits while negative by preliminary and confirmatory procedures) and one case showed pseudo benzodiazepines positive results (positive results by Investigated kits benzodiazepines while it was positive for opiates by preliminary and confirmatory procedures) and one case showed pseudo benzodiazepines negative results (negative results by Investigated kits for benzodiazepines and positive for barbiturates while, it was positive for benzodiazepines by preliminary and confirmatory procedures).
Benzodiazepine detection is complicated. Not due to a large number of benzodiazepines, the number of metabolites potentially present amplifies the number of reactant molecules with various levels of cross reactivity [16].
Wiencek et al. [1] documented that the antibodies incorporated into IAs are subject to cross reactivity with other compounds. Cross-reactions can occur with drugs of similar chemical structure, but can also occur with totally unrelated drugs. So, IA results should be considered presumptive positive until confirmed by a more specific method, like gas chromatography/mass spectrometry (GC/MS) or high-pressure liquid chromatography/ mass spectrometry (HPLC/MS).
In the present work, the validity tests were accepted for both types, I and II investigated kits as regards sealing, container material, white cup tap, creatinine parameter, nitrite parameter, glutaraldehyde, pH, specific gravity and oxidant/pcc.
Most of rapid tests contain one or more testing strips for an individual drug [17].When validating devices containing multiple testing strips, each drug strip should be considered a separate individual test. This can be a challenge as some testing devices now on the market have 10–12 different drug tests in a single kit [18]. Some of these devices can also detect common methods of adulteration such as dilution, bleach, nitrites, or pH. Even though these rapid kits offer the convenience of rapid turnaround time and have a wide array of drug class detection, many problems can be avoided by performing a thorough investigation of the device performance prior to implementation.
Several methods can detect sample adulteration including changes in sample appearance, the presence of color or bubbles (Mary Jane Super Clean 13-lemon-scented dish detergent), changes in sample pH (Amber13 and bleach), alterations in sample specific gravity, or the presence of a specific adulterant like nitrites (Klear or Whizzies), glutaraldehyde (Urine Aid) or pyridinium chlorochromate (Sweet Pea’s Spoiler) [6]. Adulterant specific tests are available in dipstick format and have also been incorporated into some rapid drug test kits [5].
Wiencek et al. [1] concluded that antibodies are subject to crossreactivity making false-positive results possible; also patients can attempt to adulterate or substitute their specimens to generate false-negative results. These limitations should be considered if action is taken on an initial drug screening result without waiting for confirmation testing. Newer POCT kits have the capability of detecting adulterants in urine samples, and oral fluid offers the advantage of observed collections without privacy concerns in order to minimize the potential for adulteration.
Drugs of abuse accepted with accuracy 100% for tetrahydricanabinol, opiate, benzodiazepines, tramadol and with accuracy 99.75% for amphetamines for type I investigated kits while, they accepted for type II investigated kits with accuracy 100% for tetrahydrocanabinol and opiate, 94.6% for benzodiazepines and need testify for tramadol and 99.25 % for amphetamines.
Wiencek et al. [1] a limitation inherent in all urine drug testing is the possibility of sample adulteration or substitution. The simplest mechanism to prevent adulteration is to have the operator observe collection, but this is not always possible or desirable due to privacy concerns. Some urine test kits contain adulterant detection for a variety of common additives and come with thermometers on the test cup. These tools are useful in detecting several common adulterants and will detect substitution with a room temperature sample.
Conclusion and Recommendation
The investigated Kits provide a rapid analysis in drug of abuse testing procedures. This is an advantage to instituting faster treatment and action in a number of settings from the emergency room, pain management, and psychiatric counseling, to suspected drugged driving and court-ordered rehabilitation.
Both Investigated Kits have the capability of detecting adulterants in urine samples, offers the advantage of observed collections without privacy concerns in order to minimize the potential for adulteration. The test can be more variable than central laboratory testing because of the variety of operators involved in the testing process.
The rapid DOA Investigated Kits can improve patient management because of the faster result and provide forensic on-site documentation of drug ingestion provided that operators realize the limitations of the test and wait for follow-up confirmation testing as appropriate before definitive action. The breadth of drug classes and reliability of the testing will certainly expand in the future due to its convenience and portability.
However, no test is fool proof, and the Investigated Kits have their few points of limitations. Antibodies are subject to crossreactivity making false-positive results possible. These limitations should be considered if action is taken on an initial drug screening result without waiting for confirmation testing.
Rapid tests can improve patient management because of the faster result and provide forensic on-site documentation of drug ingestion provided that operators realize the limitations of the test and wait for follow-up confirmation testing as appropriate before definitive action.
Conflict of Interests
The authors declare that they have no competing interests.
Funding
Non-funded work.