Research - (2022) Volume 8, Issue 7
Effect of Transforming Growth Factor-β2 on Smad-Independent Signaling in MC3T3-E1 Cells
Hiroyuki Komamura1,
Akira Nakajima1,2*,
Remi Sano1 and
Mitsuru Motoyoshi1,2
1Department of Orthodontics, Nihon University, Japan
2Dental Research Center, Nihon University, Japan
*Correspondence:
Akira Nakajima, Department of Orthodontics, Nihon University,
Japan,
Email:
Received: 01-Jul-2022, Manuscript No. IPBMBJ-22-14021;
Editor assigned: 03-Jul-2022, Pre QC No. IPBMBJ-22-14021(PQ);
Reviewed: 17-Jul-2022, QC No. IPBMBJ-22-14021;
Revised: 22-Jul-2022, Manuscript No. IPBMBJ-22-14021(R);
Published:
29-Jul-2022, DOI: 10.36648/2471-8084-22.8.81
Abstract
The aim of this study was to investigate the effects of bone formation on the Smad-independent signaling pathways in MC3T3-E1 cells stimulated with recombinant human TGF-β2 (rhTGF-β2). The concentration of TGF-β2 in cells subjected to an optimal compressive force of 1.0 g/cm2 (simulate as orthodontic force) was determined using an ELISA. After cell stimulation with 2.5 ng/mL rhTGF-β2 for 3 h, the expression of Smad-independent signaling factors (ERK1/2, TAK1, p38, and JNK) and the expression of osteogenic transcriptional factors (Msx2 and Dlx5) were determined. The expression levels of phosphorylated Smad-independent signaling and transcription factors were also significantly increased by rhTGF-β2 stimulation. These increased expression levels were significantly decreased by LY2109761, an inhibitor of TGF-β receptors. Our new findings suggest that the Smad-independent pathway defines cellular-specific responses to TGF-β2. In addition, rhTGF-β2 stimulation induces the osteogenic transcription factors, Msx2 and Dlx5, via MAPK phosphorylation in the Smad-independent signaling pathway in osteoblasts.
Keywords
TGF-β2 signalling; Bone formation; Mechanical stress; Smad-independent signalling; MAPK signalling;
MC3T3-E1 cells
Abbreviations
(TGF) Transforming Growth Factor; (RH) Recombinant Human; (ELISA)
Enzyme-Linked Immunosorbent Assay; (MAPKs) Mitogen-Activated
Protein Kinases; (ERK) Ras-Raf-MEK-ERK; (TAK1) TGF-β-Activated
Kinase; (JNK) c-Jun N-TERMINAL KINASE; (p38) p38-MAPK;
(Msx2) Msh Homeobox 2; (Dlx5) Distal-Less Homeobox 5; (PCR)
Polymerase Chain Reaction
Introduction
Orthodontic tooth movement is caused by bone remodeling.
Various molecules are present in the periodontal tissue, including
alveolar bone osteoblast/osteoclast interactions, during tooth
movement [1-3]. Transforming growth factor-β (TGF-β) plays a role
in cell proliferation, migration, and apoptosis through processes
partially controlled by complex adhesive interactions between cellular receptors [3-5]. TGF-β has three main isoforms: TGF-β1,
TGF-β2, and TGF-β3 [4,6,7]. TGF-β2 is most commonly involved in
developmental processes that occur in affected bone tissues, such
as epithelial-mesenchymal interactions, cell growth, extracellular
matrix production, and remodeling [4,7,8]. The TGF-β2 null mutant
exhibits perinatal mortality and a wide range of developmental
defects due to single gene disruption [9].
Ligands of the TGF-β superfamily bind to type I and II receptors
(Tβr1/2) [4,6,7]. The type I receptors then phosphorylate receptor-
regulated Smads (signal transducers known as “mothers
against decapentaplegic homolog”) (R-Smads; e.g., Smad2 and
Smad3), which can bind to common partner Smads (Co-Smads;
e.g., Smad4) [10,11]. This Smad-complex (Smad-dependent) signaling
from TGF-β is shared by the bone morphogenetic protein
(BMP) pathway [4,6,7,10-12]. R-Smad/Co-Smad complexes accumulate
in the nucleus, where they act as transcription factors
and participate in the regulation of target gene expression via Smad-dependent signaling [4,6,7].
TGF-βs activate Smad-independent signaling cascades that induce
Smad signaling and Smad-independent TGF-β responses. The latter
activates the mitogen-activated protein kinases (MAPKs) pathway,
including the Ras-Raf-MEK-ERK (ERK) pathway, the TGF-β-activated
kinase (TAK) 1/LK3/MEKK1 pathway and the c-Jun N-terminal
Kinase (JNK) and p38-MAPK (p38) pathways [4,13,14]. JNK and
p38-MAPK activation by TGF-βs is accompanied by Tβr-kinase activity-
independent TRAF6-TAK1 phosphorylation [4]. However, the
precise Smad-independent signaling pathways involved in bone
formation are unclear.
A previous study suggested that cyclic mechanical strain results
in a significant increase in active TGF-β1 levels, with no effects on
the amount of total TGF-β1. TGF-β1 activation also leads to the
phosphorylation of Smad2-mediated transcriptional elevation
of downstream mediators and auto-induction of TGF-β1 [5]. As
a result, TGF-β2 is considered to promote the differentiation of
cells into osteoblasts to promote bone formation. Previously, we
determined the effects of compressive force on the TGF-β1 and
TGF-β2 signaling pathways [15]. Using MC3T3-E1 osteoblast-like
cells, we observed increased TGF-β1 and TGF-β2 expression levels
in cells subjected to 1.0 g/cm2 of compressive force for 3–6
h. The 1.0 g/cm2 compressive force-induced phosphorylation of
Smad2/3 (Smad-dependent signaling) and upregulation of osteogenic
transcription factors, such as Runt-related transcription
factor 2 (Runx2) and Osterix (known as Sp7 transcription factor),
were attenuated by pretreatment with a Tβr inhibitor [15]. This
finding indicated that 1.0 g/cm2 compressive force could induce
bone-specific transcription factors via the autocrine action of
TGF-β1/2 signaling in osteoblasts [15].
To date, whether the specific functional role of TGF-β2 including
the Smad-independence signaling during tooth movement is
stimulated by the optimal mechanical stress of compressive force
is unclear. Further, whether the two main Smad-independent
pathways are specified by the exact regulatory mechanism and
functions of TGF-β2 stimulation for bone formation during osteoblastic
cell function, including the expression of osteogenetic transcription
factors, such as Msh homeobox 2 (Msx2) and distal-less
homeobox 5 gene (Dlx5), is unknown. To identify the functional
role of TGF-β2 in osteoblastic function via the Smad-independent
signaling pathway (e.g., ERK1/2 and TAK1), we opted to conduct
experimental stimulation of TGF-β2 using an optimum compressive
force in the MC3T3-E1 cell line. The objective of the present
study was to determine whether osteoplasty is performed via the
Smad-independent signaling pathway with TGF-β2 under an optimal
compressive force.
Materials and Methods
Cell Culture and Enzyme-Linked Immunosorbent
Assay (ELISA)
MC3T3-E1 cells from a mouse calvarial cell line were obtained from the RIKEN Bio Resource Center (Tsukuba, Japan) and used as
osteoblast-like cells. The cells were maintained in α-minimal essential
medium (α-MEM; Gibco BRL, Rockville, MD, USA) containing
10% (v/v) heat-inactivated fetal bovine serum (FBS; HyClone
Laboratories, Logan, UT, USA) at 37°C in a humidified atmosphere
containing 95% air and 5% CO2. Cells were subjected to 1.0 g/
cm2 optimal compressive force for 3 h, based on a previous report
that described elevated TGF-β2 expression under these conditions
[15]. Thereafter, the amounts of TGF-β2 in the culture medium
were determined using an ELISA kit (R and vD Systems, Minneapolis,
MN, USA) according to the manufacturer’s instructions. The assays
were performed in triplicate for each specimen, and the data
were converted to ng/mL [2]. To evaluate the TGF-β2 downstream
signaling pathway, cells were seeded in a 100 mm2 dish at a density
of 1.0 × 104 cells/cm2 and treated for up to 3 h with 2.5 ng/
mL recombinant human (rh) TGF-β2 (R and D Systems, Inc. Minneapolis,
MN, USA), based on the ELISA quantitation. Cells treated
without rhTGF-β2 were used as a control.
Cell Culture with Exogenous Tβr1/2 Inhibitor
(LY2109761)
LY2109761, an orally active Tβr1/2 kinase dual inhibitor (I, Ki=38
nmol/L; II, Ki=300 nmol/L [15]) was used to indicate Smad-independent
signaling of TGF-β2. MC3T3-E1 cells were seeded in
6-well cell culture dishes at a density of 1.0 × 104 cells/cm2. After
overnight incubation, the cells were treated for up to 3 h with 10
nM and 25 nM exogenous LY2109761 (G-T, Minneapolis, MN) 30
minutes before rhTGF-β2 stimulation.
Real-Time Reverse Transcription Polymerase
Chain Reaction (RT-PCR)
Total mRNA was isolated from cultured MC3T3-E1 cells using a
commercially available kit (RNeasy Mini Kit; Qiagen, Valencia, CA).
Aliquots containing equal amounts of mRNA were subjected to real-
time RT-PCR. First-strand cDNA synthesis was carried out using
1 μg of DNase-treated total mRNA in 20 μL of a solution containing
first-strand buffer, 50 ng random primers, 10 mM dNTP mixture, 1
mM DTT, and 0.5 U reverse transcriptase at 42°C for 60 min. The
cDNA mixtures were diluted five-fold using sterile distilled water,
and 2-μL aliquots were subjected to real-time RT-PCR using SYBR
Green I dye. Real-time RT-PCR was performed using a 25 μL final
reaction volume containing 1X R-PR buffer, 1.5 mM dNTP mixture,
1X SYBR Green I, 15 mM MgCl2, 0.25 U ExTaq polymerase real-time
RT-PCR version (TaKaRa, Tokyo Japan), and 20 mM specific primers
(TaKaRa, Tokyo Japan); the primer sequences are listed in (Table 1).
PCR was performed using a thermal cycler (Smart Cycler, Cepheid,
and Sunnyvale, CA) and the data were analyzed using Smart Cycler
software (ver. 1.2d). The cycling conditions were 95°C for 3 s and
68°C for 20 s for 35 cycles. Measurements were performed at the
end of the annealing step at 68°C in each cycle. The specificity of
the RT-PCR products was verified by adding melting curve analysis
between 68 and 94°C. All real-time RT-PCR runs were performed
in triplicate, and the mRNA expression levels were calculated and
normalized to the level of GAPDH mRNA [1,2].
Table 1: Primer sequences used for PCR.
| Primer |
Forward |
Reverse |
GenBank A/c No. |
| Msx2 |
5’-TGCAAGCGGCATCCATATACA-3’ |
5’-GCGTGGCATAGAGTCCCACA-3’ |
NM_013601 |
| Dlx5 |
5'-CCGCTTTACAGAGAAGGTTTCA-3' |
5-TCTTCTTGATCTTGGATCTTTTGTT-3' |
NM_010056 |
| GAPDH |
5’-CAATGACCCCTTCATTGACC-3’ |
5’-GACAAGCTTCCCGTTCTCAG-3’ |
XM_001473623 |
Western Blot Analysis
To obtain whole-cell extracts, MC3T3-E1 cells were cultured with
or without rhTGF-β2 stimulation, rinsed with phosphate buffered
saline, and then exposed to a lysis buffer comprising 50 mM Tris–
HCl, 0.1% Triton X-100, 0.1 mM EDTA, and 1 mM phenylmethylsulfonyl
fluoride. Cells in lysis buffer were sonicated three times (10
s each time). Aliquots containing equal amounts of protein were
subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE).
Conditioned medium from western blots was collected, and aliquots
containing equal amounts of protein were analyzed by SDSPAGE.
Samples were loaded on 10–12% polyacrylamide gels and
transferred to PVDF membranes using a semidry transfer unit. The
membranes were probed with anti-p-TAK1, anti-p-ERK1/2, p-p38,
and p-JNK (Cell Signaling Technology Japan, Tokyo, Japan; dilution
1:1000, polyclonal rabbit antibodies), anti-Msx2 and anti-Dlx5
(Santa Cruz Biotechnology, CA, USA; dilution 1:500), or anti-GAPDH
antibodies as internal standards (Millipore, MA, USA; monoclonal
mouse antibody, dilution 1:500) followed by a biotin-conjugated
secondary antibody (Invitrogen, CA, USA; dilution 1:10,000). The
membranes were then treated with horseradish peroxidase-conjugated
streptavidin. Immunoreactive proteins were visualized
using a chemiluminescence kit (Amersham Life Science, Buckinghamshire,
UK), according to the manufacturer’s instructions. The
intensities of the blots were quantified using a computer scanner
(Epson PX-603F, Seiko Epson, Tokyo, Japan) and digital image analysis
software (Scion Image, version Beta 4.0.3, Scion Corporation,
NIH, USA) [16].
Statistical Analysis
Statistical analysis was conducted using the statistical Package for
Social Sciences (version 8.0 for Windows, SPSS Japan Inc, Tokyo,
Japan). Student’s t-test was used to compare differences between
the control and 1.0 g/cm2 compressive force groups for the enzyme-
linked immunosorbent assay results. The other data of
Western blot and real-time revers transcription polymerase chain
reaction for the statistical analysis were performed one-way analysis
of variance (ANOVA) followed by of Tukey’s honestly significant
difference tests using for multiple comparison between each data.
Statistical significance was defined as a P-value of less than 0.05.
Results
Effect of Compressive Force on TGF-β2 Expression
In a previous study, TGF-β2 expression in MC3T3-E1 cells occurred
at an optimal compressive force of 1.0 g/cm2 for 3 h [15]. In order
to identify Smad-independent pathway via a specific TGF-β2 signaling,
the amount of TGF-β2 in MC3T3-E1 cells subjected to the
optimum force was determined using ELISA. Compared with the
control group (not subjected to compressive force), the concentration
of TGF-β2 was detected 2.54 ng/mL (Figure 1).
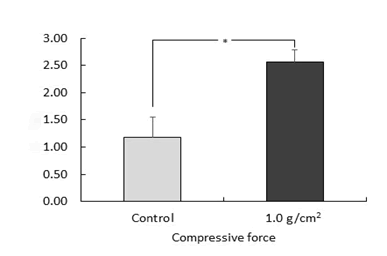
Figure 1: Expression of TGF-β2 in cells subjected to 1.0 g/cm2 of compressive force
MC3T3-E1 cells were cultured with or without a continuous compressive
force of 1.0 g/cm2 for up to 3 h. After 24 h, TGF-β2 protein
levels in cells was measured from the culture medium using an
enzyme-linked immunosorbent assay (n=5, *p<.05).
To confirm the stimulation induced by rhTGF-β2 and the effect
of the Tβr1/2 inhibitor (LY2157299), western blot analysis was performed. After stimulation with rhTGF-β2, the expression of
TGF-β2 was significantly increased compared to that in the control.
This increased TGF-β2 expression was significantly decreased
by LY2157299 in a dose-dependent manner (Figure 2).
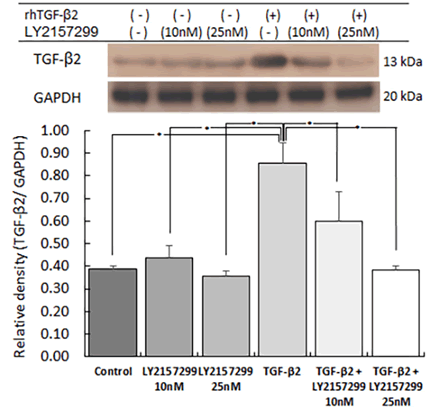
Figure 2: Effect of rhTGF-β2 stimulation and Tβr inhibition on TGF-β2 expression
To confirm the stimulation of recombinant human (rh) TGF-β2 (2.5
ng/mL) and the effect of the TGF-β receptor inhibitor (LY2157299;
10 nM or 25 nM), western blot analysis was performed. The expression
of TGF-β2 was significantly increased compared to that in
the control after stimulation with rhTGF-β2. This increased TGF-β2
expression was significantly decreased by LY2157299 in a dose-dependent
manner (n=5, *p<.05).
Effect of ERK1/2 Phosphorylation by TGF-β2 Stimulation
Smad-independent signaling mainly involves two MAPKs pathways,
one of which is the ERK1/2 cascade [4,17]. The phosphorylation
of ERK1/2 was found to be significantly increased by rhTGF-
β2 stimulation (p<0.05). In fact, this increase in expression
was approximately 1.5-fold higher than that found in the control.
Phosphorylation of ERK1/2 was also decreased by LY2109761 in a
dose-dependent manner (p<0.05) (Figure 3).
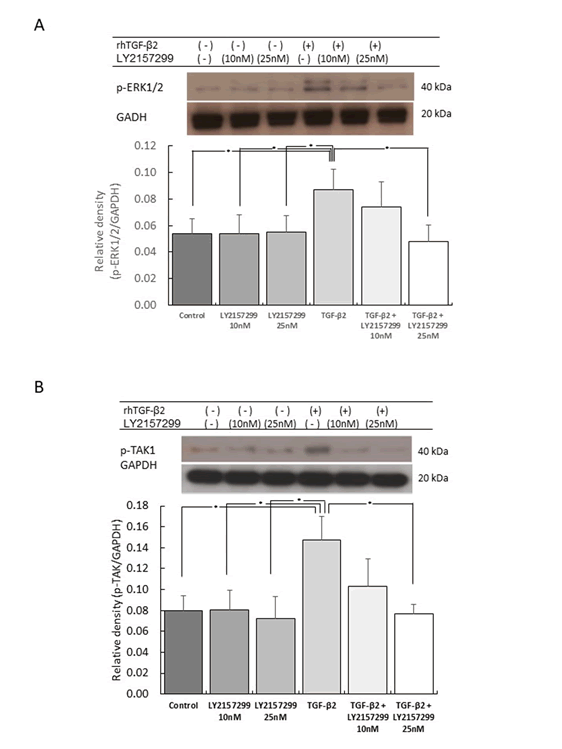
Figure 3: Effect of rhTGF-β2 stimulation and Tβr inhibition on ERK1/2 and TAK1 phosphorylation
The expression of phosphorylated ERK1/2 (p-ERK1/2; A) and TAK1
(p-TAK1; B) was significantly increased by recombinant human
TGF-β2 stimulation. p-ERK1/2 and p-TAK1 expression levels were
approximately 1.5 and 1.8-fold higher, respectively, than that in
the control (n=5, *p<0.05). This upregulation was significantly reduced
by the Tβr inhibitor LY2157299 in a dose-dependent manner.
Thus, the relative density of p-ERK1/2 expression in MC3T3-E1
cells was significantly increased by stimulation with rhTGF-β2
compared to that in control cells.
Effects of TAK1, p38, and JNK Phosphorylation by
TGF-β2 Stimulation
Another Smad-independent signaling pathway is the TAK1 cascade
[4,18]. The phosphorylation of TAK1 was significantly increased by
rhTGF-β2 stimulation (approximately 1.8-fold higher than that in
the control; p<0.05). The phosphorylation of TAK1 was decreased
by LY2109761 in a dose-dependent manner (p<0.05) (Figure 3B).
The downstream molecules of TAK1 signaling include p38 and
JNK [4,18]. The expression of p-p38 was significantly increased
by rhTGF-β2 stimulation, with an approximate 1.75-fold increase
compared to that in the control (p<0.05). This upregulated p-p38
expression was significantly reduced by LY2157299 in a dose-dependent
manner (Figure 4A).
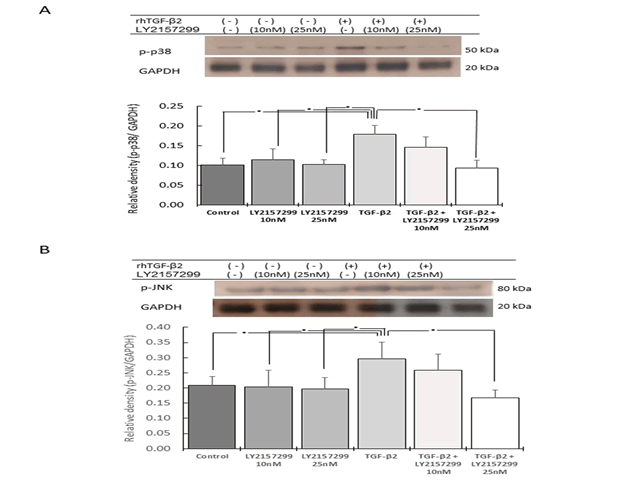
Figure 4: Effect of rhTGF-β2 stimulation and Tβr inhibition on p38 and JNK phosphorylation
The expression of p-JNK was also significantly increased by TGF-β2
stimulation. In fact, p-JNK expression was approximately 1.5-fold
higher than that in the control (p<0.05), but was decreased by the
inhibitor (LY2109761) in a dose dependent manner (p<0.05) (Figure
4B).
The phosphorylation of p38 (A) and JNK (B) was determined using
western blot analysis to identify the balance in Smad-independent
signaling. The phosphorylation of p38 (p-38) and JNK (p-JNK) was
significantly increased by rhTGF-β2 stimulation. p-p38 and p-JNK
protein levels were approximately 1.75 and 1.5-fold higher, respectively,
than those of the control (n=5, *p<0.05). Phosphorylation
was significantly decreased by the Tβr inhibitor LY2109761 in
a dose-dependent manner (n=5, *p<0.05; A and B).
Therefore, TAK1, p38, and JNK phosphorylation were increased by
TGF-β2 stimulation, and decreased by 25 nM LY2157299, leading
to the same level found for the control.
Changes in the Expression of Msx2 and Dlx5
To identify the expression of osteogenic transcription factors following
rhTGF-β2 stimulation and/or LY2157299 inhibition, the
mRNA expression of Msx2 and Dlx5 was determined using real-
time PCR and the protein levels were observed using western
blot. Msx2 gene (Figure 5A) and protein (Figure 5B) expression
levels were significantly increased compared with those of the
control (p<0.05). Further, this increased expression was significantly
decreased by LY2157299.
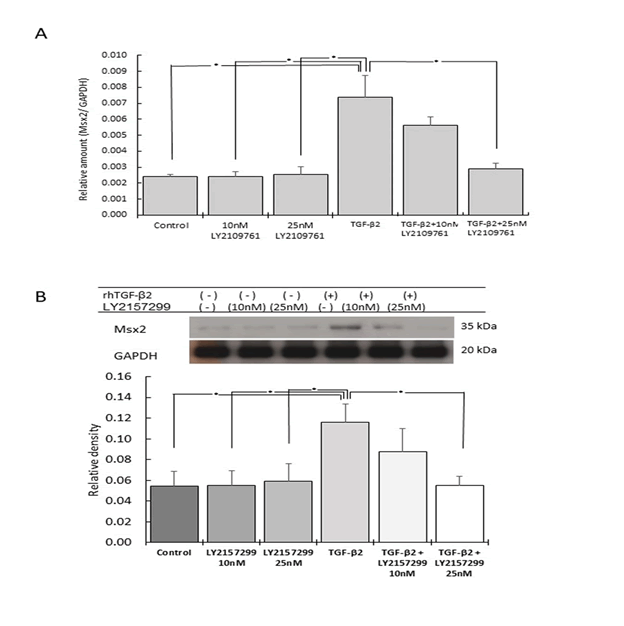
Figure 5: Effect of rhTGF-β2 stimulation and Tβr inhibition on Msx2 gene and protein expression
The gene (A) and protein (B) expression levels of Msx2 were significantly
increased by rhTGF-β2 stimulation, but significantly reduced
by the Tβr inhibitor LY2157299 (n=5, *p<0.05).
Dlx5 expression was significantly increased in cells stimulated
with rhTGF-β2 compared to control cells (p<0.05) (Figures 6A and
6B). The Dlx5 gene and protein expressions were also significantly
reduced by LY2157299. These increased expression levels may
not be influenced by the Smad-dependent signaling pathway, but
may be mediated via the Smad-independent pathway. Therefore,
these osteogenic transcription factors could be associated with
the TGF-β2 signaling pathway, which could be mediated via the
Smad-independent pathway.
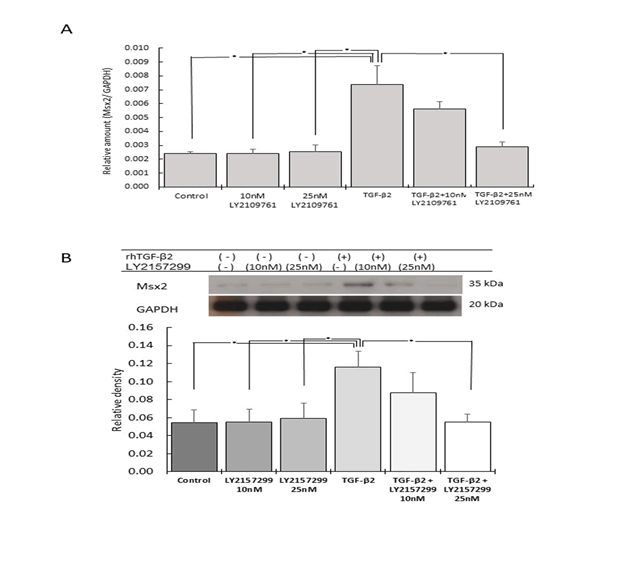
Figure 6: Effect of rhTGF-β2 stimulation and Tβr inhibition on Dlx5 gene and protein expression
The gene (A) and protein (B) expression levels of Dlx5 were significantly
increased by rhTGF-β2 stimulation and decreased by the Tβr
inhibitor LY2157299 in a dose-dependent manner (n=5, *p<0.05).
Discussion
The Smad-independent effects of rhTGF-β2 stimulation upon
compressive force were evaluated in MC3T3-E1 cells and the expression
level of osteogenic transcription factors owing to TGF-β2
signaling was determined. To accurately demonstrate the relationship
between TGF-β2 and the Smad-independent signaling
pathways using compressive force, the amount of TGF-β2 was first
determined. Thereafter, the cells were stimulated using a specific
amount of rhTGF-β2. Consequently, TGF-β2, which is expressed
due to optimum compressive force, and TGF-β2 stimulation in osteoblasts
could clarify the influence of Smad-independent signaling
and the expression of osteogenic transcription factors.
In terms of MAPK signaling, osteoblast differentiation and bone
formation may be influenced by the Smad-independent TGF-β
signaling pathway [11,18,19]. The association of TAK1 (known
as MAP3K7) is induced by TGF-β2, resulting in the activation of
the JNK or the MAPK-p38 signaling cascades. TAK1 regulates the
steady-state protein levels of these three kinases [11,18]. According to a previous study, exposure to either dynamic or static pressure
induces the initial osteogenic differentiation of mesenchymal
stem cells [14]. Particularly, both types of pressure strongly stimulated
the expression of osteogenesis-related factors in undifferentiated
mesenchymal stem cells. Extracellular signal-regulated
kinases (ERKs) signaling participates in early osteo-differentiation and plays a positive but non-critical role in mechano-transduction
[14]. Indeed, the present results indicate that TGF-β inhibition
prevents rhTGF-β2-induced phosphorylation of ERK1/2 and
TAK1 expression. Further, the current data support the notion that
Smad-independent signaling can promote osteoblast differentiation
via the expression of osteogenesis-related transcription factors.
Following Smad-independent phosphorylation, the expression of
Msx2 and Dlx5 as osteogenetic transcription factors was induced
by an effect exhibited by the TGF-β2 specific signaling pathways.
As a result, their expression levels were significantly increased by
rhTGF-β2 stimulation. Msx2 and Dlx5 are homeobox genes that
encode homeodomain protein products that share a characteristic
protein fold structure that binds DNA to regulate the expression
of target genes [20-23]. Homeodomain proteins regulate gene
expression and cell differentiation during early embryonic development;
therefore, mutations in homeobox genes can cause developmental
disorders [21,23,24].
Msx2 is localized on human chromosome 5, which encodes a transcriptional
repressor and activator responsible for craniofacial and
limb-bud development [23]. Cells express Msx2 when exposed to
signaling molecules, such as BMP-2 and BMP-4, which are members
of the TGF-β family [24,25]. The expression of Msx2 leads
to the proliferation, migration, and osteogenic differentiation of
neural crest cells during embryogenesis and bone fracture. Germline
knockout mice have been created for this gene (Msx2 ±) to
enable evaluations of functional loss [26]. Msx2 null mice exhibit
intramembranous and endochondral ossification defects [27] and
a decreased number of chondrocytes in their resting, proliferating
and hypertrophic zones. In our study, the cell numbers in these
three zones decreased, suggesting that specific TGF-β2 signaling is
involved in chondrogenesis by controlling Msx2 expression in undifferentiated
cells that induce endochondral bone.
As previously mentioned, Dlx5 is a member of the homeobox transcription factor gene family [20-22]. Previous studies have shown
that the homeobox gene family is important for appendage development
[21]. DLX5 is necessary for proper craniofacial, axial,
and appendicular skeleton development [21,22]. A previous study
found that the expression of Dlx5 is enhanced in Tgfbr2fl/fl; Wnt1-
Cre mice and that its deletion resulted in a partial rescue of the
cartilage phenotypes [21]. Therefore, we propose that TGF-β2 and
Dlx5 induce osteoblastic functions via Smad-independent signaling,
which determines the optimal compressive force stimulation.
Based on the relation between mechanical stress and the expression
of Msx2 and Dlx5, which are osteogenic transcription factors,
a previous study reported that a compressive force of 1.0 g/cm2 significantly increased the mRNA and protein expression of Msx2
and Dlx5, which are critical for osteoblast differentiation in the
osteoblastic cell line (ROS 17/2.8 cells) [28]. In the present study,
the expression of these osteogenesis-related transcription factors
could also be strongly associated with the TGF-β2 signaling pathway
via a Smad-independent signaling cascade. Interestingly, a
recent study demonstrated that, following TGF-β induction, both
TAK1 and ERK MAPK pathways converge at Msx2 and Dlx5 in their
control of mesenchymal precursor cell differentiation [29]. Future
studies should investigate the expression of osteogenic transcription
factors via the TGF-β2 signaling pathway during the inhibition
of either ERK1/2 or TAK1 signaling. As TGF-β can have positive or negative regulatory effects on osteoblast differentiation [3,30,31],
our findings suggest that rhTGF-β2 is appropriate for promoting
osteogenesis.
Conclusion
rhTGF-β2 stimulated the expression of components of the Smad-independent
TGF-β signaling pathway, including osteogenic transcription
factors, such as Msx2 and Dlx5, in MC3T3-E1 cells. These
osteoblastic differentiation phenomena were reflected by specific
Smad-independent signaling pathway molecules, suggesting that
TGF-β2 stimulation induces osteogenic bone formation, at least
under the present experimental conditions. Overall, the results of
this study could be used to specify the TGF-β2 signaling pathway
under optimum compressive force, and the Smad-independent
pathways may define cellular-specific responses to TGF-β2.
Acknowledgements
The authors are thankful to their colleagues at the Department of
Orthodontics and the Department of Oral Health Sciences, Nihon
University School of Dentistry, for their continuous support.
Author Contributions
The contributions of all authors are described in the following
statement:
1. Study conception and design of study: Remi Sano, Akira Nakajima,
and Mitsuru Motoyoshi.
2. Analysis and interpretation of results: Hiroyuki Komamura,
Remi Sano, Akira Nakajima and Mitsuru Motoyoshi.
3. Draft manuscript preparation: Hiroyuki Komamura, Remi
Sano, Akira Nakajima and Mitsuru Motoyoshi.
4. The results and approved the final version of the manuscript:
Hiroyuki Komamura, Remi Sano, Akira Nakajima and Mitsuru
Motoyoshi.
Declaration of Competing Interests
Declarations of interest: none
Funding
This work was supported by JSPS KAKENHI Grant Number
JP22K10280, and the Dental Research Center in Nihon University
School of Dentistry, Graduate School of Dentistry (1 and 2).
REFERENCES
- Mitsui N, Suzuki N, Maeno M, Yanagisawa M, Koyama Y, et al. (2006) Optimal compressive force induces bone formation via increasing bone morphogenetic proteins production and decreasing their antagonists production by Saos-2 cells. Life Sci 78 (23):2697-706.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, Koyama Y, Sanuki R, Mitsui N, Suzuki N, et al. (2010) IL-17A stimulates the expression of inflammatory cytokines via celecoxib-blocked prostaglandin in MC3T3-E1 cells. Arch Oral Biol 55 (9):679-88.
[Crossref] [Google Scholar] [PubMed]
- Manokawinchoke J, Limjeerajarus N, Limjeerajarus C, Sastravaha P, Everts V, et al. (2015) Mechanical force-induced tgfb1 increases expression of sost/postn by hpdl cells. J Dent Res 94 (7):983-9.
[Crossref] [Google Scholar] [PubMed]
- Nakajima A, Shuler FC, Gulka AOD, Hanai JI (2018) TGF-beta signaling and the epithelial-mesenchymal transition during palatal fusion. Int J Mol Sci 19 (11)
[Crossref] [Google Scholar] [PubMed]
- Peters AS, Brunner G, Krieg T, Eckes B (2015) Cyclic mechanical strain induces TGFbeta1-signalling in dermal fibroblasts embedded in a 3D collagen lattice. Arch Dermatol Res 307 (2):191-7.
[Crossref] [Google Scholar] [PubMed]
- Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, et al. ( 2018) tgf-beta family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol 10 (5).
[Crossref] [Google Scholar] [PubMed]
- Wu M, Chen G, Li YP (20160 TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009.
[Crossref] [Google Scholar] [PubMed]
- Takeyama K, Chatani M, Inohaya K, Kudo A (2016) TGFbeta-2 signaling is essential for osteoblast migration and differentiation during fracture healing in medaka fish. Bone 86:68-78.
[Crossref] [Google Scholar] [PubMed]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, et al. (1997) TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124 (13):2659-70.
[Crossref] [Google Scholar] [PubMed]
- Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM (2015) TGF-beta/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res 3:15005.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Deng C, Li YP (2012) TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8 (2):272-88.
[Crossref] [Google Scholar] [PubMed]
- Zhao S, Nan L, Wang Y, Wei L, Mo S (2021) Effects of Smad4 on the expression of caspase3 and Bcl2 in human gingival fibroblasts cultured on 3D PLGA scaffolds induced by compressive force. Int J Mol Med 47(3).
[Crossref] [Google Scholar] [PubMed]
- Li J, Zhao Z, Yang J, Liu J, Wang J, X. Li, et al. (2009) P38 MAPK mediated in compressive stress-induced chondrogenesis of rat bone marrow MSCs in 3D alginate scaffolds. J Cell Physiol 221 (3):609-17.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Zhao Z, Li J, Zou L, Shuler C, Zou Y, et al. (2009) Hydrostatic pressures promote initial osteodifferentiation with ERK1/2 not p38 MAPK signaling involved. J Cell Biochem 107 (2):224-32.
- Sano R, Nakajima A, Kawato T, Maeno M, Shimizu N (2017) Effect of Compressive Force on TGF- β1/2 Signaling Pathway in MC3T3-E1 Cells. J Hard Tissue Biol 26(2):177–186.
[Crossref] [Google Scholar]
- Nakajima A, Ito Y, Tanaka E, Sano R, Karasawa Y, et al. (2014) Functional role of TGF-beta receptors during palatal fusion in vitro. Arch Oral Biol 59 (11):1192-204.
[Crossref] [Google Scholar] [PubMed]
- Shen J, Li S, Chen D (2014) TGF-beta signaling and the development of osteoarthritis. Bone Res 2 :14002.
[Crossref] [Google Scholar] [PubMed]
- Chen IT, Hsu PH, Hsu WC, Chen NJ, Tseng PH (2015) Polyubiquitination of transforming growth factor beta-activated kinase 1 (tak1) at lysine 562 residue regulates tlr4-mediated jnk and p38 mapk activation. Sci Rep 5:12300.
[Crossref] [Google Scholar] [PubMed]
- Thielen NGM, van der Kraan PM, van Caam APM (2019) TGFbeta/BMP signaling pathway in cartilage homeostasis. Cells 8 (9).
[Crossref] [Google Scholar] [PubMed]
- Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, et al. (1999) Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 126 (17):3795-809.
[Crossref] [Google Scholar] [PubMed]
- Oka K, Oka S, Hosokawa R, Bringas P Jr, Brockhoff HC, et al. (2008) TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev Biol 321 (2):303-9.
[Crossref] [Google Scholar] [PubMed]
- Robledo RF, Rajan L, Li X, Lufkin T (2002) The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev 16 (9):1089-101.
[Crossref] [Google Scholar] [PubMed]
- Hosokawa R, Urata M, Han J, Zehnaly A, Bringas P Jr, et al. (2007) TGF-beta mediated Msx2 expression controls occipital somites-derived caudal region of skull development. Dev Biol 310 (1):140-53.
[Crossref] [Google Scholar] [PubMed]
- Alappat S, Zhang ZY, Chen YP (2003) Msx homeobox gene family and craniofacial development. Cell Res 13 (6):429-42.
[Crossref] [Google Scholar] [PubMed]
- Rifas L, Towler DA, Avioli LV (1997) Gestational exposure to ethanol suppresses msx2 expression in developing mouse embryos. Proc Natl Acad Sci U S A 94 (14):7549-54.
[Crossref] [Google Scholar] [PubMed]
- Melville H, Wang Y, Taub PJ, Jabs EW ( 2010) Genetic basis of potential therapeutic strategies for craniosynostosis. Am J Med Genet A 152A (12):3007-15.
[Crossref] [Google Scholar] [PubMed]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, et al. (2000) Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24 (4):391-5.
[Crossref] [Google Scholar] [PubMed]
- Yanagisawa M, Suzuki N, Mitsui N, Koyama Y, Otsuka K, et al. (2008) Compressive force stimulates the expression of osteogenesis-related transcription factors in ROS 17/2.8 cells. Arch Oral Biol 53 (3):214-9.
[Crossref] [Google Scholar] [PubMed]
- Lee KS, Hong SH, Bae SC (2002) Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene 21 (47):7156-63.
[Crossref] [Google Scholar] [PubMed]
- Luttrell LM, Dar MS, Gesty-Palmer D, El-Shewy HM, Robinson KM, (2019) Transcriptomic characterization of signaling pathways associated with osteoblastic differentiation of MC-3T3E1 cells. PLoS One 14 (1):e0204197.
[Crossref] [Google Scholar] [PubMed]
- Suzuki E, Ochiai-Shino H, Aoki H, Onodera S, Saito A, et al. (2014) Akt activation is required for TGF-beta1-induced osteoblast differentiation of MC3T3-E1 pre-osteoblasts.PLoS One 9 (12):e112566.
[Crossref] [Google Scholar] [PubMed]
Citation: Komamura H, Nakajima A, Sano R, Motoyoshi M (2022) Effect of Transforming Growth Factor-?2 on Smad-Independent Signaling in MC3T3-E1 Cells. Biochem Mol Biol J. 8:81.
Copyright: © 2022 Komamura H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source
are credited.







