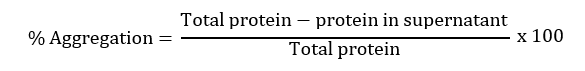Research Article - (2023) Volume 9, Issue 2
Differential Proteolytic Stabilities of Soluble Proteins and Amyloid Like Protein Aggregates Isolated from Human Cataract Eye Lenses
Chandrika Mittal1,
Ram Swaroop Harsolia2 and
Jay Kant Yadav1*
1Department of Biotechnology, Central University of Rajasthan, Ajmer, Rajasthan, India
2Department of Ophthalmology, Jawaharlal Nehru Medical College and Hospital, Rajasthan, India
*Correspondence:
Jay Kant Yadav, Department of Biotechnology, Central University of Rajasthan,
Ajmer, Rajasthan,
India,
Email:
Received: 16-Sep-2022, Manuscript No. IPJECS-22-14442;
Editor assigned: 19-Sep-2022, Pre QC No. IPJECS-22-14442 (PQ) ;
Reviewed: 04-Oct-2022, QC No. IPJECS-22-14442;
Revised: 03-Feb-2023, Manuscript No. IPJECS-22-14442 (R);
Published:
10-Feb-2023, DOI: 10.21767/2471-8300-9.2.021
Abstract
Background: The cross β-sheet structures in amyloids are usually proteolytic resistant. The amyloid like protein aggregates derived from human cataracts are distinct in the sense that they do not exist as fibrils rather they are found to be aggregates of sequestered crystalline.
Methods: The proteolytic susceptibility was measured by recording changes in the Congo Red (CR) bathochromic shifts, Thioflavin T (ThT) and 8-Anilino-1-Naphthalene Sulfonic acid (ANS) fluorescence emission. The difference between the degradation patterns of these two fractions of the isolated protein was further estimated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Furthermore, to substantiate the data regarding the proteolytic degradation of aggregated crystalline, the native PAGE has been performed which provided an evident confirmation of its degradation.
Findings: The obtained data suggest that the soluble protein is less susceptible to proteolytic cleavage than the insoluble amyloid like protein aggregates.
Conclusions: The data suggest that these aggregates are highly susceptible to proteolytic degradation; hence, it is inferred that they do not exist in a conventional amyloid like conformation.
Keywords
Amyloid; Cataract; Crystallin; Proteolytic cleavage, Trypsin
Introduction
The loss of nifty vision due to the opacification of the eye lens is considered to be one of the most usual causes, estimating the affected number of 16 million people in the overall world. This phenomenon of opacification of the eye lens is known as the cataract with the increase in age, many risk factors are identified, such as ultraviolet light exposure, genetic composition and diabetes [1-3]. Some evidence claims the presence of amyloid structures in the human cataract tissue, while other shows the presence of amorphous aggregates only [4,5]. As the presence of amyloid in cataracts has been seen in vitro, its presence in vivo has still been supported with little pieces of evidence [6]. While the spectroscopic data has observed the presence of amyloid β-sheets in mature lenses and cataracts, the TEM images have not detected the fibrillary structures of lens tissue [7]. Apart from that, also the corresponding contribution of the amyloid like secondary structures in the formation or the progression of cataracts remains poorly explained and less defined [8]. It is already known that conventional amyloids tend to exhibit various unusual properties like protease and detergent resistance the ability to induce aggregation in the soluble form of protein and interaction with specific dyes [9,10]. For example, some of these tend to be less sensitive to the action of proteases in comparison to the native form of the same protein [11]. Similarly, these features have also been illustrated for those protein aggregates that lack the cross-β structure, generally known as ‘amyloid-like’, which are diverse in many ways. The proteins which get misfolded and interact fallaciously generate the proteolysis-resistant fibrils or aggregates known as amyloids. The primary structures of these proteins are different, however, in their amyloid genic form they possess a unique cross beta-sheet structure. These cross-beta sheet structures are responsible for the fibrillary, insoluble and protease-resistant nature of these proteins [12,13]. One of the most common examples of this theory is the Aβ fibrils. These are known to hinder the digestion by proteases like bovine brain proteases and trypsin, as well as the aggregates of α-synuclein, which were also observed to impede the degradation by cat [14,15]. Therefore, the assessment of proteolytic resistance of these fibrillary structures is utilized as an effective approach nowadays to study the influence or effect of external factors on the structure and stability of these amyloids [16]. Some studies have already shown that the susceptibility of protein towards aggregation, low structural stability and protease degradation, as contributing factors to cataract development [17]. Since the formation of aggregates in the cells of the eye lens has been known to resemble the mechanism of self-assembly carried by amyloid fibrils in neurodegeneration the presence of non-fibrillary amyloid like aggregates is evident in the cataract lenses no homogeneity has been observed in cataract amyloids to the classic amyloids [18,19]. Therefore, the current study aims to investigate the proteolytic resistance of the amyloid like protein isolated from human cataract eye lenses. Whether they show any resemblance to the conventional amyloids regarding the proteolytic resistance or not. Also, a comparative proteolytic susceptibility would be able to provide deeper insight into their molecular structure. Both the insoluble and soluble fractions of proteins have been subjected to trypsin digestion, to probe the difference between the two fractions of the same protein.
Materials and Methods
Bovine Serum Albumin (BSA), Potassium Dihydrogen Phosphate (KH2PO4), Sodium Chloride (NaCl), Disodium Hydrogen Phosphate (Na2HPO4), tris-base, Potassium Chloride (KCl) acrylamide/bisacrylamide, sodium dodecyl sulfate, pH paper, ethanol, Sodium Hydroxide (NaOH) and Phenyl Methyl Sulfonyl Fluoride (PMSF) were acquired from the HiMedia Laboratories Pvt. Ltd., Mumbai, India. Ethylene Diamine Tetraacetic Acid (EDTA), Bradford reagent, lysozyme, trypsin (porcine pancreas) Congo Red (CR), Calcium Chloride (CaCl2) 8-Anilino-1-Naphthalenesulfonic Acid (ANS), Thioflavin T (ThT) and Sodium Azide (NaN3), were acquired from the sigma-aldrich chemical company, USA.
The Insoluble and Soluble Protein Isolation from the Cataract Lenses of Humans
The department of ophthalmology, of the JLN Medical College and Hospital, Ajmer, Rajasthan, India, the cataract eye lenses from human patients were collected. After collection, by following the guidelines of the institutional ethical committee of JLN medical college and hospital (Ref. No. 1557/Acad.- III/MCA/2016) and the Indian council of medical research, New Delhi, the samples were treated and stored. The lenses were brought in the cryovials to the laboratory under a cold environment, dipped in 1 × DPBS buffer, containing 30% w/v trehalose and 10% DMSO and kept in a -80°C freezer. A total number of 5-6 lenses were taken and brought to room temperature by thawing continuously. All the lenses were washed with 3 ml to 4 ml of the chilled tri-calcium buffer (pH 7.5), after being transferred into a 50 ml centrifuge tube for 30 minutes on ice. Lenses were subjected to blot drying with the help of a blotting paper and transferred to another centrifuge tube. The sample was then immersed in 4 ml to 6 ml, of the tris-EDTA buffer (pH 7.5), following manual homogenization. The homogenate was centrifuged at 4°C for 30 minutes, at 9500 rpm. The supernatant obtained after the centrifugation contains the soluble protein of the eye lens, which was then stored at 4°C for further use. The pellet was suspended in 3 ml to 4 ml of the ice-cold autoclaved distilled water and homogenized well. The sample was then centrifuged at 9500 rpm for 30 minutes at 4°C, to isolate the insoluble protein aggregates. Subsequently, the supernatant was collected in another 50 ml centrifuge tube and the pellet was homogenized in 3 ml to 4 ml of the ice-cold autoclaved distilled water. The homogenized sample was again centrifuged 6 to 7 times by using the above-mentioned steps. Afterward, all the collected supernatants were pooled in a single falcon. The final pellet was dissolved in 1 × DPBS buffer and considered cell debris. Subsequently, 10 mM EDTA and 0.2 M NaCl were added to the obtained supernatant which was then incubated at 4°C for 3 days. After incubation, the supernatant was centrifuged at 4°C for 30 minutes, at 9500 rpm. The pellet was taken and resuspended in 400 μl to 500 μl of ice-cold autoclaved distilled water. This suspension of the pellet is considered as insoluble protein aggregates (amyloidlike aggregates), of the human cataract eye lens 20.
Proteolytic Cleavage of the Insoluble and Soluble Protein of the Cataract Eye Lens of Human
To assess the proteolytic susceptibility of the isolated insoluble and soluble protein, a stock solution of 1 mg/ml trypsin was prepared by dissolving trypsin in distilled water. The soluble and insoluble protein fractions were mixed with the trypsin solution in the ratio of 10:1 (Protein:Trypsin), by keeping the protein and trypsin concentrations to 0.5 mg/ml and 0.05 mg/ml, respectively. After mixing, samples were incubated at 37°C for 3 hours at 800 rpm. To stop the enzymatic reaction after the completion of incubation time, 0.1 mM PMSF which was prepared by dissolving PMSF in isopropanol, was added to the samples 21. Similarly, the same protocol has been accounted for the proteolytic digestion of lysozyme aggregates, taken as a positive control for proteolytic resistant amyloid aggregates [20-23].
Percentage Aggregation
The effect of hydrolysis on the insoluble protein by trypsin has been estimated by analyzing the percentage aggregation of both protein samples. The protein samples were centrifuged at 4°C for 10 minutes at 14000 rpm. The supernatant was taken out and protein concentration was estimated by using Bradford analysis. After getting the protein concentration, the percent aggregation of the samples was calculated by using the below formula:
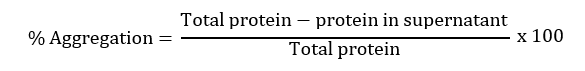
Congo Red (CR) Binding Assay
CR is an amyloid-specific histologic dye, which contains a high affinity toward the cross β- sheet structures of protein aggregates. It binds to the amyloid-specific regions of proteins. This binding leads to a rise in the absorption spectra, providing a redshift of the maximum absorption in wavelength. The CR stock solution of 0.1% w/v was prepared by dissolving the dye in distilled water. The CR binding assay for different samples of protein was performed by keeping the CR and protein sample concentrations to 35 μM and 250 μg/ml, respectively. By using the ELISA plate reader, the absorption spectra were recorded between 400 nm to 600 nm, by mixing the protein and CR in an appropriate amount following incubation of 10 minutes [24,25].
Thioflavin T (ThT) Fluorescence Assay
ThT molecule is known for its efficacy in detecting amyloid fibrils as a molecular probe. For the analysis of samples, we prepared a ThT stock solution of 200 μM in distilled water. The samples to be characterized for amyloid presence were mixed with this ThT solution, by keeping the final concentrations at 0.25 mg/ml and 20 μM, respectively. After the incubation of 15 minutes in dark, the ThT fluorescence spectra were recorded in the range of 470 nm to 650 nm, after the excitation at 450 nm. The spectra were recorded using a JASCO fluorimeter in a quartz cuvette having a path length of 1 cm the width of the slit for the excitation and emission was fixed at 10 nm and 5 nm, respectively.
8-Anilino-1-Napthalenesulfonic Acid (ANS) Fluorescence Assay
To detect the protein’s hydrophobic clusters, ANS binding assay is used as it specifically binds to these clusters. This method is used widely to probe any kind of conformational changes that take place within protein molecules. For the analysis of samples, we prepared a stock solution of ANS (200 μM) in distilled water. The protein samples to be characterized for amyloid presence were mixed with the ANS solution, by keeping the final concentrations at 0.25 mg/ml and 20 μM, respectively. After the incubation of 15 minutes in dark, ANS fluorescence, in the range of 400 nm to 670 nm was recorded, after the excitation at 355 nm. The spectra were recorded using a JASCO fluorimeter in a quartz cuvette having a path length of 1 cm. The width of the slit for the excitation and emission was fixed at 10 nm and 5 nm, respectively.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
To obtain more knowledge about the banding patterns we performed the SDS-PAGE of the protein samples. A 10% resolving gel was prepared, by mixing the acrylamide/bisacrylamide solution with 10% sodium dodecyl sulfate, 1.5 M tris-HCl (pH:8.8), 10% ammonium persulfate and TEMED, in distilled water, in an appropriate amount. A stacking gel of 5% was prepared by mixing the acrylamide/bis-acrylamide with 10% sodium dodecyl sulfate, 1 M tris-HCl (pH:6.8), 10% ammonium persulfate and TEMED in distilled water, in an appropriate an amount. Sample preparation was performed by mixing the sample buffer with protein samples in an equal volume according to the required concentration. The prepared samples were heated for 5 minutes at 95°C. Prepared gels were then mounted in the electrophoretic apertures and 1 × electrophoresis buffer was poured into the SDS-PAGE unit tank. In each well of the prepared gel, 20 μl of protein samples were loaded and the electric current was switched on. For stacking gel, the electric current was set at 80 V and for resolving gel, it was set at 100 V. After the sample got fully resolved, the gel was gently taken out and followed by its rinsing with distilled water. The rinsed gel was then transferred to the staining solution and incubated for 20-25 minutes. Afterward, the stained gel was then transferred to the de-staining solution and kept on a gel rocker for agitation. The de-stained gel was immersed in distilled water and stored for visualization by using the gel doc.
Native-Polyacrylamide Gel Electrophoresis (Native Page)
Subsequently, the native page of the untreated and trypsintreated insoluble protein was performed, along with the native soluble protein isolated from the human cataract lens. The protocol used for the SDS-page was implemented for performing the native page, excluding the involvement of protein denaturing agents like SDS and β-mercaptoethanol. The heat denaturation step was also excluded from the practice.
Results and Discussion
Primarily, the effect of hydrolysis was analyzed by estimating the percentage aggregation of the samples. As shown in Figure 1, a decrease in the aggregation percentage is observed in the trypsin-treated insoluble protein.
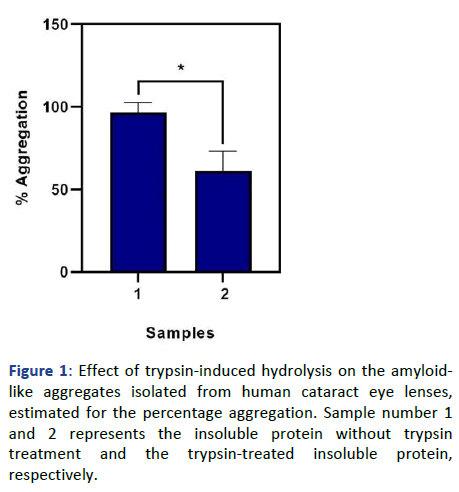
Figure 1: Effect of trypsin-induced hydrolysis on the amyloidlike aggregates isolated from human cataract eye lenses, estimated for the percentage aggregation. Sample number 1 and 2 represents the insoluble protein without trypsin treatment and the trypsin-treated insoluble protein, respectively.
Subsequently, all the samples were examined for the presence or absence of amyloidogenicity, by using conventional amyloid-specific techniques (CR binding assay and ThT fluorescence assay). The CR absorption assay has provided the spectrum having λmax at 485 nm of CR alone. The untreated insoluble protein and trypsin digested insoluble protein have provided a spectral shift of 9 nm and 1 nm from CR control, respectively. In Figure 2, as a significant decrease in the CR shift has been observed in the trypsin digested insoluble protein, we can say that the trypsin has shown some activity upon the aggregates, as the decrease in the amyloidogenicity of the aggregated protein is observed.
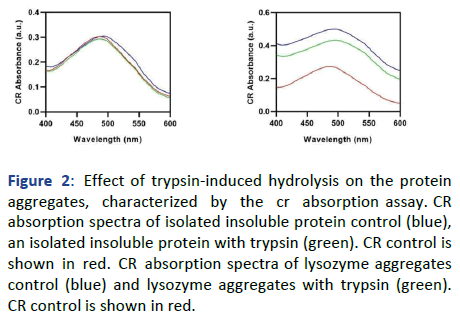
Figure 2: Effect of trypsin-induced hydrolysis on the protein aggregates, characterized by the cr absorption assay. CR absorption spectra of isolated insoluble protein control (blue), an isolated insoluble protein with trypsin (green). CR control is shown in red. CR absorption spectra of lysozyme aggregates control (blue) and lysozyme aggregates with trypsin (green). CR control is shown in red.
Similarly, in Figure 3, a significant decrease has been observed in the peak of trypsin treated insoluble protein aggregates of the cataract eye lens, whereas, the untreated insoluble protein aggregates have shown the highest fluorescence intensity.
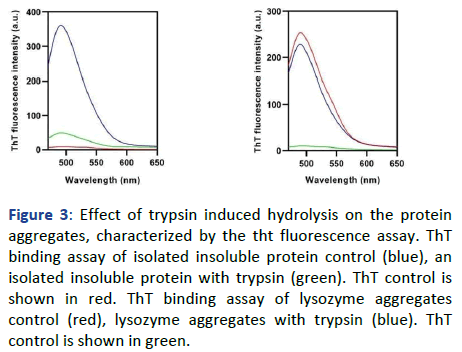
Figure 3: Effect of trypsin induced hydrolysis on the protein aggregates, characterized by the tht fluorescence assay. ThT binding assay of isolated insoluble protein control (blue), an isolated insoluble protein with trypsin (green). ThT control is shown in red. ThT binding assay of lysozyme aggregates control (red), lysozyme aggregates with trypsin (blue). ThT control is shown in green.
As the binding of ThT molecules to the amyloid aggregates is the hallmark characteristic of this assay, it can be inferred that the amyloidogenicity of the aggregates has decreased. Whereas, the trypsin digested lysozyme aggregates have not shown any significant decrease in the CR absorbance shift and ThT fluorescence intensity, respectively. Due to this, it can be inferred that the aggregates of lysozyme persist in the form of amyloid aggregates in it. Subsequently, the ANS binding assay has also been performed to obtain information regarding the surface hydrophobicity of the trypsin treated and untreated insoluble aggregates isolated from human cataract eye lenses. As Figure 4 shows, the untreated insoluble protein aggregate has the highest ANS fluorescence intensity in place of the trypsin-digested insoluble aggregate. As the ANS binding to the hydrophobic pockets of the protein aggregates is the hallmark characteristic of this technique, it can be inferred that after the trypsin-digestion, the aggregates have gone through some degradation due to which the surface hydrophobicity has been decreased the surface hydrophobicity of the lysozyme aggregates did not show any significant decrease upon trypsin treatment, which was assessed by ANS fluorescence intensity.
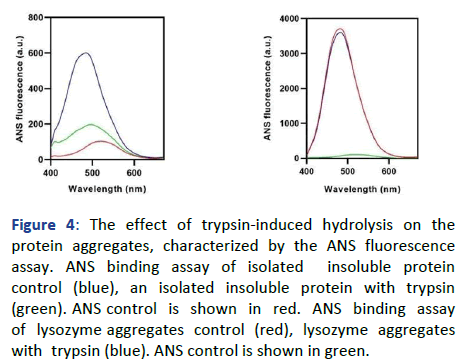
Figure 4: The effect of trypsin-induced hydrolysis on the protein aggregates, characterized by the ANS fluorescence assay. ANS binding assay of isolated insoluble protein control (blue), an isolated insoluble protein with trypsin (green). ANS control is shown in red. ANS binding assay of lysozyme aggregates control (red), lysozyme aggregates with trypsin (blue). ANS control is shown in green.
To further substantiate the data, the SDS-PAGE of the samples has been performed to generate information regarding the change in the banding patterns of the proteins. As Figure 5 shows, the insoluble protein without trypsin digestion has not shown prominent bands in the SDS-PAGE, rather most of the protein got stuck in the stacking gel which confirms the, highly stable aggregated form of the protein. Whereas, the trypsin digested insoluble fraction has got fully resolved in the gel providing a prominent banding pattern similar to soluble protein.
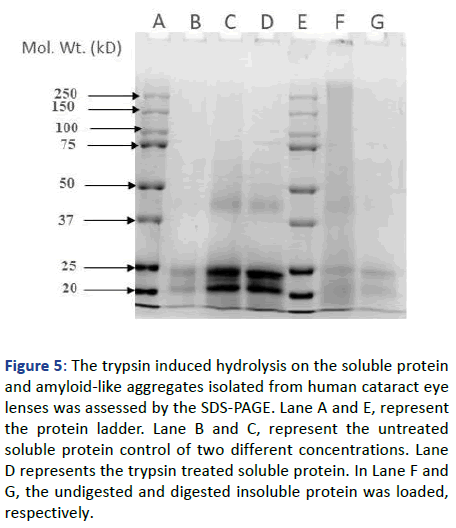
Figure 5: The trypsin induced hydrolysis on the soluble protein and amyloid-like aggregates isolated from human cataract eye lenses was assessed by the SDS-PAGE. Lane A and E, represent the protein ladder. Lane B and C, represent the untreated soluble protein control of two different concentrations. Lane D represents the trypsin treated soluble protein. In Lane F and G, the undigested and digested insoluble protein was loaded, respectively.
This suggests that the insoluble fraction has gone through degradation due to which it got resolved in the gel without leaving any trace in the stacking gel. Similarly, the SDS-PAGE of the trypsin treated and untreated soluble protein has also been performed to check the similarity and dissimilarity in the effect of protease on both the fractions of protein. The trypsin treated soluble protein has got easily resolved into the gel providing bands similar to the untreated one. Following these banding patterns, we can say that the trypsin has not digested the protein in its soluble or native form, as it has not provided any further bands that might have come due to the truncation of protein into smaller fragments. Furthermore, to support the above hypothesis regarding the proteolytic susceptibility of cataracts amyloid, the native PAGE of the untreated and trypsin-treated insoluble protein was performed. Along with these two samples, the native soluble protein isolated from the human cataract lens was also taken. This was performed to differentiate between the treated and non-treated insoluble protein based on the resemblance of banding patterns towards the native soluble protein. By the observed banding pattern, as shown in Figure 6, it can be inferred that the insoluble protein aggregates had undergone the digestion or hydrolysis process up to an extent, upon treatment with trypsin, as some amount of protein had got resolved in the gel and provided a similar smudge of a band like soluble protein.
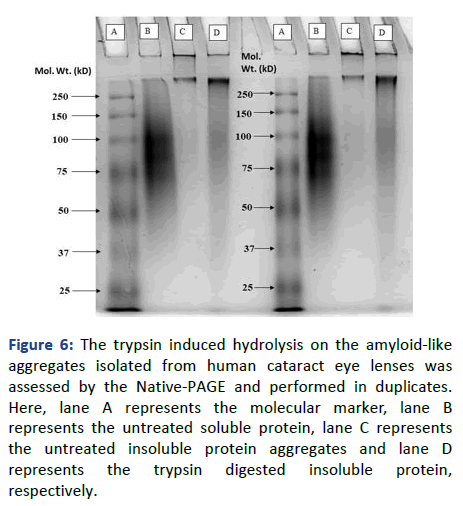
Figure 6: The trypsin induced hydrolysis on the amyloid-like aggregates isolated from human cataract eye lenses was assessed by the Native-PAGE and performed in duplicates. Here, lane A represents the molecular marker, lane B represents the untreated soluble protein, lane C represents the untreated insoluble protein aggregates and lane D represents the trypsin digested insoluble protein, respectively.
The insoluble fraction of the protein isolated from the human cataract eye lens has been assessed for its proteolytic stability. Here, it was found that the protein aggregates treated with trypsin have shown solubilization up to an extent. In compliance with the above data, we can say that the amyloid like protein aggregates isolated from human cataract eye lenses are not proteolytic resistant like the other conventional amyloids formed in vivo. The soluble fraction is rather proven to possess proteolytic resistance. Furthermore, to justify the hypothesis, the obtained results were compared with the lysozyme aggregates, taken as a positive control of proteolytic resistant amyloid aggregates. From the above results, the proposed hypothesis can be supported and can be utilized in the generation of a possible pathway for the events which might have taken place in the proteolytic degradation/ hydrolysis of crystallin protein aggregates, as shown in Figure 7.
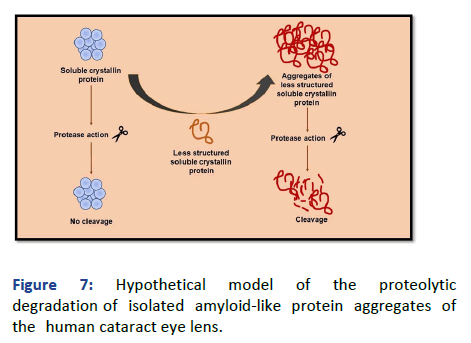
Figure 7: Hypothetical model of the proteolytic degradation of isolated amyloid-like protein aggregates of the human cataract eye lens.
Conclusion
Structurally, the fibrils are made up of intermolecular crossbeta structures. These cross beta structures are composed of beta-sheets and beta-strands that are parallel and perpendicular to the fibril axis, respectively. Generally, the amyloid structure which is adopted by only a part of the protomer forms the core and the stem of the amyloid. The other remaining part of the protomer is attached to the stem in a very flexible manner. It can retain the structure and some function of its original form, if it is represented by a separate functional domain. Due to these structural properties, the amyloid cores generally attain increased proteolytic resistance, separating them biochemically from the other regions of these protomers. In this study, the proteolytic resistance of the insoluble amyloid like aggregates has been assessed. After the trypsin digestion, the number of aggregates and amyloidogenicity of the insoluble fraction decreased significantly, as shown by the percentage aggregation data, amyloid-specific stain-based techniques (CR and ThT), SDS PAGE and native PAGE, respectively. The surface hydrophobicity of the trypsin digested insoluble aggregates has also decreased significantly, as shown by the ANS binding assay, by which the degradation of the aggregates by trypsin treatment can be inferred. From the obtained data it can be concluded that the amyloid like protein aggregates isolated from human cataract eye lenses differ in the structural organization from the classic amyloids, as they had shown susceptibility against protease. Subsequently, by SDS-PAGE, we can say that the soluble fraction of the protein isolated from human cataract eye lenses has shown proteolytic resistance whilst subjected to trypsin digestion. Furthermore, the results obtained from native PAGE support this hypothesis, as the degradation of the insoluble aggregates into smaller fragments has been observed in the gel. Hence, structural differences can be observed in both the fractions of isolated proteins.
Funding
The work was financially supported by the research grant (Ref. No. EMR/2017/005417), sponsored by the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India, New Delhi, India.
Competing Interests
All authors declare no conflict of interest.
References
- AsbellPA, Dualan I, Mindel J, Brocks D, Ahmad M, (2005) Age related cataract. Lancet. 365(9459):599-609. [Crossref][Google Scholar] [PubMed]
- SchaumbergDA, Dana MR, Christen WG, Glynn RJ (1998) A systematic overview of the incidence of posteriorcapsule opacification. Ophthalmology. 105(7):1213-1221. [Crossref][Google Scholar] [PubMed]
- Nagai N, ManoY, Otake H, Shibata T, Kubo E, et al. (2017) Amyloid β 1-43 accumulates in the lens epithelium of corticalopacification in Japanese patients. Invest Ophthalmol Vis Sci. 58(7):3294-3320. [Crossref] [Google Scholar] [PubMed]
- Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE (2004) Amyloid Fibril Formation by Lens Crystallin Proteins andIts Implications for Cataract Formation. J Biol Chem. 279(5):3413-3419. [Crossref][Googlescholar][Indexed]
- Chemerovski-GlikmanM, Mimouni M, Dagan Y, Haj E, Vainer I, et al. Rosmarinic acid restores complete transparency of sonicatedhuman cataract ex vivo and delays cataract formation in vivo. Sci Rep. 8(1):9341. [Crossref][Google Scholar] [PubMed]
- Alperstein AM, Molnar KS, Dicke SS, Farrell KM, Makley LN, et al. (2021) Analysis of amyloid-like secondary structure in theCryab-R120G Knock-in mouse model of hereditary cataracts by two-dimensionalinfrared spectroscopy. PLoSOne. 16(9):0257098. [Crossref][Google Scholar] [PubMed]
- Alperstein AM, Ostrander JS, Zhang TO, Zanni MT (2019) Amyloid found in human cataracts with two-dimensionalinfrared spectroscopy. ProcNatl Acad Sci U S A. 116(14):6602-6607. [Crossref] [Google Scholar] [PubMed]
- Ecroyd H,Carver JA (2009) Crystallin proteins and amyloid fibrils. Cell Mol Life Sci. 66(2):62-81. [Google Scholar]
- Malisauskas M, Weise C, Anamandra K, Watz MW, Roche ML (2010) Lability landscape and protease resistance of humaninsulin amyloid: A new insight into its molecular properties. J Mol Biol. 396(1):60-74. [Crossref][Google Scholar] [PubMed]
- Matiiv AB,Trubitsina NP, Matveenko AG, Barbitoff YA, Zhouravleva GA, et al. (2020) Amyloid and amyloid likeaggregates: Diversity and the term crisis. Biochemistry (Moscow). 85(9):1011-1034. [Crossref][Google Scholar] [PubMed]
- Kushnirov VV, Dergalev AA, Alexandrov AI (2020) Proteinase K resistant cores of prions and amyloids. Prion. 14(1):11-19. [Crossref][Google Scholar] [PubMed]
- Glenner GG, Ein D, Eanes ED, Bladen HA, Terry W, et al. (1971) Creation of “Amyloid” Fibrils from Bence Jones Proteins in vitro. Science. 174(4010):712-714. [Crossref][Google Scholar] [PubMed]
- Ningthoujam DS, Mukherjee S, Devi LJ, Singh ES, Tamreihao K, et al. (2019) In vitro degradation of β-amyloid fibrils by microbial keratinase. Alzheimers Dement (N Y). 5:154-163. [Crossref][Google Scholar] [PubMed]
- Lambeth TR,Julian RR (2021) Proteolysis of amyloid β by lysosomal enzymes as a functionof fibril morphology. ACSOmega. 6(47):31520-31527. [Crossref][Google Scholar] [PubMed]
- McGlinchey RP, Dominah GA, Lee JC (2017) Taking a bite out of amyloid: Mechanistic insights into α-synucleindegradation by Cathepsin L. Biochemistry. 56(30):3881-3884. [Crossref][Google Scholar] [PubMed]
- Soderberg L, Dahlqvist C, Kakuyama H, Thyberg J, Ito A, et al. (2005) Collagenous alzheimer amyloid plaque component assemblesamyloid fibrils into protease resistant aggregates. FEBS J. 272(9):2231-2236. [Crossref][Google Scholar] [PubMed]
- Liu J, XuW, Wang K, Chen F, Ren L, et al. (2022) Congenital cataract causing mutation ΒB1-L116P is prone toamyloid fibrils aggregation and protease degradation with low structural stability. Int J Biol Macromol. 195:475-482. [Crossref][Google Scholar] [PubMed]
- Clark JI (2013) Self-assembly of protein aggregates in ageing disorders: The lens and cataract model. hilosTrans R Soc Lond B Biol Sci. 368(1617):20120104. [Crossref][Google Scholar] [PubMed]
- Boatz JC, Whitley MJ, Li M, Gronenborn AM, Wel VD (2018) Cataract-associated P23T ΓD-crystallin Retains anative-like fold in amorphous looking aggregates formed at physiological PH. Nat Commun. 8(1):15137. [Crossref] [Google Scholar] [PubMed]
- Tennent GA (1999) Isolation and characterization of amyloid fibrils from tissue. Methods Enzymol. 309:26-47. [Crossref][Google Scholar] [PubMed]
- Stepanenko OV, Sulatsky MI, Mikhailova EV, Stepanenko OV, Kuznetsova IM, et al. (2021) Trypsin induced degradation of amyloid fibrils. Int J Mol Sci.22(9):4828. [Crossref][Google Scholar] [PubMed]
- Scialo C, De Cecco E, Manganotti P, Legname G (2019) Prion and prion-likeprotein strains: Deciphering the molecular basis of heterogeneity in neurodegeneration. Viruses. 11(3):261. [Crossref] [Google Scholar] [PubMed]
- Wickner R, Son, M, Edskes H (2019) Prion variantsof yeast Are numerous, mutable and segregate on growth, affecting prionpathogenesis, transmission barriers and sensitivity to anti-prion systems. Viruses. 11(3):238. [Crossref] [Google Scholar] [PubMed]
- Kushnirov VV, Dergalev AA, Alexandrov AI (2020) Proteinase K resistant cores of prions and amyloids. Prion. 14(1):11-19. [Crossref][Google Scholar] [PubMed]
- Rambaran RN,Serpell LC (2008) Amyloid fibrils. Prion. 2(3):112-117. [Crossref] [Google Scholar] [PubMed]
Citation: Mittal C, Harsolia RS, Yadav JK (2023) Differential Proteolytic Stabilities of Soluble Proteins and Amyloid-like Protein Aggregates Isolated from Human Cataract Eye Lenses. J Eye Cataract Surg. 9:21
Copyright: © 2023 Mittal C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.