Keywords
Biomass smoke; COPD; miR-10-5p; miR-15b-5p; miR-30d; miR-200c-
5p; Smoking
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as
a preventable and treatable disease characterized by airflow
limitation that is not fully reversible [1]. The main risk factor
for the development of COPD is smoking (TS) [1]; however, in
developing countries such as Mexico, women are mainly affected
by domestic exposure to biomass smoke (BS) [2,3]. COPD from
biomass smoke (COPD-BS) is considering a phenotype distinct
from that of COPD from smoking (COPD-TS). The distinctive
prognostic factors between both pathologies are widely described
[2-4]. Thus, women with COPD-BS remain mostly in stages I-II of COPD, evolving infrequently to stages III-IV, contrary to those
with COPD-TS who most commonly evolve toward stages III-IV
of COPD [2,3].
Several factors interact in the origin and development of the pathological process in COPD, including genetic, epigenetic,
biochemical, physiological and environmental components [5].
Based on epigenetic factors, alterations in the expression of
microRNAs (miRNAs or miRs) is essential [6]. miRNAs are small
non-coding RNAs that silence and regulate posttranscriptional
modification of mRNAs. Thus, certain miRNA expression profiles
have been associated with the pathogenesis of various diseases
[6,7]. miRNAs can be secreted by cell damage or lysis, and
transported in body fluids in a stable manner, encapsulated or
anchored in microvesicles, exosomes, lipoproteins or Argonaute-2
protein [7,8].
The profile analysis of extracellular miRNAs has been used as a
tool to monitor the physiopathological state, by shown a good
correlation with disease states and/or risk conditions [6-8].
Thus, for example, five circulating unregulated miRNAs were
found in the serum of patients with COPD-TS, with four of them
underexpressed: miR-20, miR-28-3p, miR-34c-5p, and miR-100;
while miR-7 was overexpressed, when compared with healthy
individuals [8]. Nevertheless, it is important to denote that those
involved in COPD-BS have not been identified. In this sense,
recently, our study group published a work analyzing which
miRNAs participate in COPD-BS compared with COPD-TS in mildto-
moderate states (GOLD I-II) [9]. Nevertheless, the miRNAs that
could be involved in COPD in severe to very severe stages (GOLD
III-IV) have not been described. Accordingly, just we published a
partial report from the same protocol performed in the present
work, which showed that women with COPD-BS mith a higher
values of DLCOsb%P compared to COPD-TS in severe to very
severe stages, based in the fact of that serum miR-22-3p was
underexpressed, concomitantly to a higher concentration of serum
the HDAC4, positively correlated with DLCOsb%P; considering
that the miR-22-HDAC4-DLCO axis favored the development of
chronic bronchitis over that emphysema in COPD-BS [10]. So
that, in the present study we show more complete comparisons,
considering 5 groups of women; women with COPD from smoking
and biomass, women exposed to biomass or smoking without
COPD, and heathy control women; focus in the comparative
analysis in the expression of serum miRNAs. We hypothesize that
COPD-BS as represents a different physio pathogenic phenotype
with respect to COPD-TS, the profile of serum miRNAs present
in women with COPD-BS, in severe to very severe COPD (GOLD
stage III-IV), will be different from those with COPD-TS, and also
with those healthy women exposed to BS (H-BS) or smoking (HTS),
and control women (C). Therefore, the objective in this study
was to compare the serum expression of serum miRNAs in these
five groups of women.
Research Methodology
Study population
This cross-sectional study was conducted in accordance with the
Declaration of Helsinki, and was approved by the Science and
Bioethics Committee at the Instituto Nacional de Enfermedades
Respiratorias Ismael Cosío Villegas (INER) in Mexico City (protocol
INER: B15-15), a referral center of respiratory diseases in Mexico.
All participants answered a written questionnaire and received and provided informed consent to participate in the study,
agreeing to protocol approved by the Committees.
A total of 125 women divided into five groups of 25 were
integrated in this study. Women were recruited at the COPD clinic
at the INER, from a cohort that was followed regularly, from May
2015 to May 2017; all participants received complete information
about the study and gave their informed consent for the study,
and data were recollected by medical doctors previously trained
on the questionnaire assessment. Women with COPD from
biomass (COPD-BS), smokers with COPD (COPD-TS), all with
COPD in severe to very severe COPD (GOLD stages III-IV), smokers
without COPD (H-TS), exposed biomass without COPD (H-BS) and
healthy control women (C).
Demographic, anthropometric, and clinical data were collected
including TS history (>10 packs-years) and cumulative exposure
to BS (h-year). No patient with COPD was exposed to both factors.
Wood was the only fuel used by women with COPD-BS, who came
from rural and suburban, low-income regions of Mexico. Were
excluded women with a history of any other chronic pulmonary
or non-pulmonary conditions, exacerbation 6 months, or lower
respiratory tract infection 4 weeks prior to study enrollment.
Pulmonary function testing
COPD was classified according to the exposure history following
the procedures recommended by the American Thoracic Society/
European Respiratory Society [11], and the standard reference for
Mexicans [10]. The pulmonary function test applied to all women
to diagnose COPD was pre- and post-bronchodilator spirometry.
Forced expiratory volume in the 1st sec % predicted (FEV1%p),
forced vital capacity % predicted (FVC%P), and FEV1/FVC ratio
were measured. Tests were done using a Sensormedics dry seal
spirometer (Yorba, Linda, CA, USA).
Blood samples
From each woman, blood samples were collected in anticoagulantfree
tubes (5mL) (BD vacutainer; Becton, Franklin Lakes, NJ,
USA), at morning with at least 8 fasting hours. The samples were
centrifuged at 5,000g at room temperature for 15 min to obtain
the serum and tested the same day. For continuous storage, the
serum was aliquoted and frozen at -80°C until use.
Obtaining serum miRNAs
The extraction of the miRNAs was performed using the QIAGEN
miRNeasy serum/plasma kit (Hilden, Germany) following the
manufacturer’s instructions. The miRNA was quantified, and
integrity was assessed with the Agilent Bioanalyzer 2100 system
(Agilent Technologies, Santa Clara, CA, USA).
Differential expression of miRNAs in serum by
PCR arrays
Serum miRNAs analysis was conducted in two stages: a screening
stage to identify miRNAs differentially expressed in a samples
from 3 women randomly selected from each study group. Once
miRNAs differentially expressed were identified, we implemented
the validation stage in 25 women of each group, using quantitative
real-time PCR (RT-qPCR).
The differential expression analysis was performed for 96 miRNAs
using MiScript miRNA PCR Array (MIHS-106Z; Qiagen, Valencia,
CA, USA), isolated from serum. After obtaining all raw results, the
data was analyzed in the Qiagen software (Data analysis file for
miScript miRNA PCR Array All miRNA QC; Qiagen, Valencia, CA,
USA) (available at https://www.qiagen.com/us/shop/genes-andpathways/data-analysis-center-overviewpage/). The software
uses the 2-ΔCt method, which analyzed and compared two
specific groups using Student’s t-test. The data is expressed as
fold change = relative quantification of miRNA.
Validation of samples by RT-qPCR
The validation was performed by RT-qPCR, obtaining the cDNA of
the miRNAs extracted with the RT kit and amplified with TaqMan
Universal Master Mix II with the UNG kit, all from Applied
Biosystems by Thermo Fisher Scientific (USA). Pre-designed
commercial assays for each miRNA were obtained from Thermo
Fisher Scientific: hsa-miR-10a-5p (ID 002288), hsa-miR-15b-5p
(ID 000390), hsa-miR-30d-5p (ID 000420), and hsa-miR-200c-5p
(ID 002300). The expression level of each miRNA was evaluated
using the comparative threshold cycle method (2-ΔΔCt) and
normalized with a corresponding miRNA sequence from C.
elegans as an exogenous normalizer in gene expression (spikein
cel-miR-39). The relative concentration of each miRNA was
described by the equation ΔCt = (Ct miRNA-Ct spike). The cutoff
value was set as the cycle ≤40 and it was considered that a
gene was not detectable when the Ct was >40 and the signal was
under established limits [12].
Statistical analysis
To obtain the sample size, the free software G Power (version
3.1.9.2; Heinrich-Heine-Universität, Düsseldorf, Germany) was used. The change in gene expression was reported in multiples
(fold-change). The statistical analysis for qPCR array was perform
with the Qiagen software (available at https://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overviewpage/).
RT-qPCR was analyzed by relative quantification (ΔΔCt
method). The analyses were performed using the statistical
package GraphPad (Graph-Pad v6.01 Software, Inc., La Jolla, CA,
USA). P< 0.05 were considered significant.
Results
Characteristics of women in the study
The demographic, anthropometric, and clinical data of the study
groups are shown in Table 1. The women with COPD-BS were
older and shorter, showing differences with the other groups (P
<0.01). Women with COPD-BS were weighed less, and showed
differences with COPD-TS and H-BS; H-TS also showed difference
with COPD-TS (P <0.01); although the BMI did not show difference
between the groups. Tobacco and biomass index did not show
difference between groups.
| Variables |
Control |
COPD-TS |
COPD-BS |
H-TS |
H-BS |
| Characteristics |
| Age (years) |
66.59 ± 7.11 |
66.57 ± 6.37 |
73.27 ± 8.69**/**//# |
63.12 ± 8.21 |
66.58 ± 7.1 |
| Height (cm) |
158.26 ± 8.10 |
155.31 ± 7.57 |
146.52 ± 5.69*/**//## |
158 ± 0.07 |
153 ± 0.1 */*//## |
| Weight (kg) |
68.55 ± 11.59 |
59.52 ± 12.13 |
56.25 ± 10.82**//## |
70.58 ± 13.46/** |
65.87 ± 12.57 |
| BMI (kg/m2) |
27.33 ± 3.91 |
24.79 ± 5.78 |
26.33 ± 5.04 |
28.16 ± 4.86 |
26.35 ± 9.01 |
| Exposure characteristics |
Tobacco index
(pack-years) |
0 |
36.62 ± 23.1 |
0 |
30.25 ± 19.26 |
0 |
Biomass smoke
Index (h-years) |
0 |
0 |
366.88 ± 219.3 |
0 |
325.87 ± 186.58 |
| Lung function characteristics |
| FEV1 %P |
96.25 ± 3.1 |
39.85 ± 5.36** |
39.92 ± 6.57** |
83.14 ± 2.3*/** |
84.32 ± 4.2*/** |
| FEV1/FVC ratio |
80.02 ± 2.4 |
45.63 ± 5.36* |
45.23 ± 10.08* |
74.12 ± 2.3*/** |
73.5 ± 2.2*/** |
| GOLD grades |
Case numbers (%) |
| III |
0 |
16 (64) |
20 (80) |
0 |
0 |
| IV |
0 |
9 (36) |
5 (20) |
0 |
0 |
The data are expressed as means ± SD. The data were analyzed by one-way ANOVA and Tukey's the post hoc test. * P<0.05, ** P<0.01, * vs. control; /** P<0.05, *P<0.01, vs. COPD-TS or COPD-BS groups; //# P<0.05; //## P<0.01, vs. H-TS or H-BS groups, respectively.
Abbreviations: BMI: Body Mass Index; C: Control Healthy Women; COPD-BS: COPD Secondary to Biomass Smoke Exposure; COPD-TS: COPD Secondary to Tobacco Smoking; FEV1% Pred: Forced Expiratory Volume in the 1st Second (% Predicted); FVC: Forced Vital Capacity; FEV1/FVC Ratio: Forced Expiratory Volume in the 1st Second (% Predicted)/ Forced Vital Capacity Ratio; H-BS: Exposed to BS Without COPD; H-TS: Smokers Without COPD.
Table 1: Anthropometric, clinical, and physiological characteristics of the study in women (n=25).
FEV1%P and FEV1/FVC ratio in both groups of women with COPD
showed differences among the H-BS, H-TS, and C groups (P
<0.01); likewise, women from the H-BS and H-TS groups showed
differences compared with both groups with COPD (p <0.01).
Additionally, with no difference between the groups with COPD.
Differential expression of miRNAs in serum by
PCR array
The differential expression analysis of the miRNAs was performed
in three patients chosen randomly from each study group. The
results obtained are shown in Table 2. Only one miRNA was overexpressed, miR-15b-5p, in women with H-TS group with the
C group. The remaining three miRNAs were underexpressed:
miR-10a-5p in women with H-TS compared with COPD-TS, hsamiR-
30d-5p in women with COPD-TS compared with the C group,
miR-200c-5p in women with COPD-BS related to those with H-BS.
| Compared groups |
miRNA |
Regulation level |
Fold change |
P-value |
| COPD-TS vs. H-TS |
miR-10a-5p |
underexpressed |
-4.10 |
0.0177 |
| H-TS vs. C |
miR-15b-5p |
overexpressed |
15.38 |
0.0236 |
| COPD-TS vs. C |
miR-30d-5p |
underexpressed |
-5.93 |
0.0060 |
| COPD-BS vs. H-BS |
miR-200c-3p |
underexpressed |
-4.15 |
0.0088 |
Samples were analyzed by PCR arrays. The data are presented as the change in regulation (fold-change) and the obtained p-value.
Abbreviations: C: Control Healthy Women; COPD-BS: COPD Secondary To Biomass Smoke Exposure; COPD-TS: COPD Secondary To Tobacco Smoking; H-BS: Exposed To BS Without COPD; H-TS: Smokers Without COPD.
Table 2: Differentially expressed miRNAs the serum of women in women with COPD from biomass exposure or smoking (GOLD stages III–IV), women
exposed to biomass or smokers without COPD, and healthy control women (n=3).
Validation of miRNAs by RT-qPCR
The four miRNAs obtained from the PCR array were subsequently
validated by RT-qPCR to corroborate the differences found
between the study groups. miR-10a-5p that was underexpressed
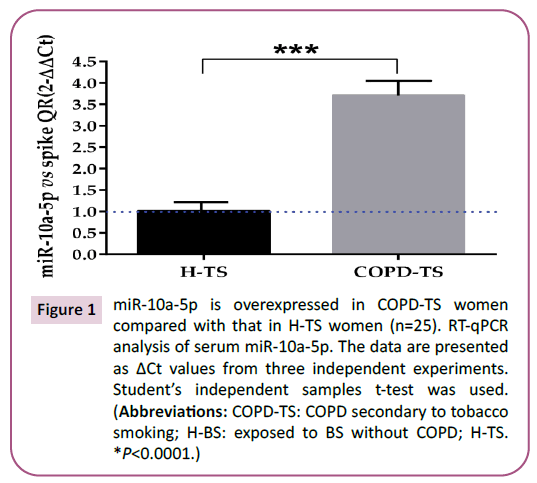
in the PCR array analysis (Table 2), was overexpressed by RT-qPCR
in women of the COPD-TS group compared with those of the
H-TS group (Figure 1; P<0.0001); similarly, miR-15b-5p that was
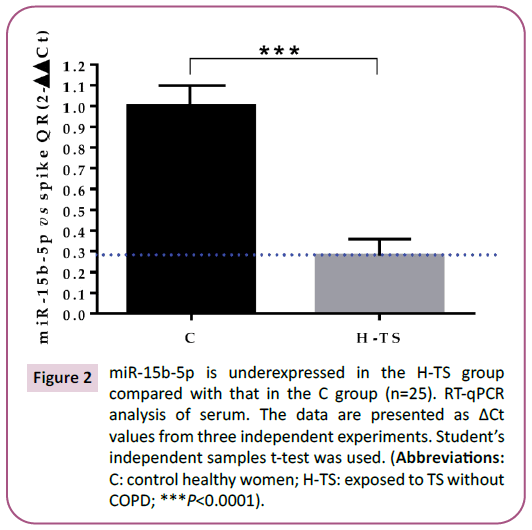
overexpressed in the PCR array and was inversely underexpressed
in the RT-qPCR among the women of the H-TS group compared
with the C group (Figure 2; P <0.0001).
Figure 1: miR-10a-5p is overexpressed in COPD-TS women
compared with that in H-TS women (n=25). RT-qPCR
analysis of serum miR-10a-5p. The data are presented
as ΔCt values from three independent experiments.
Student’s independent samples t-test was used.
(Abbreviations: COPD-TS: COPD secondary to tobacco
smoking; H-BS: exposed to BS without COPD; H-TS.
*P<0.0001.)
Figure 2: miR-15b-5p is underexpressed in the H-TS group
compared with that in the C group (n=25). RT-qPCR
analysis of serum. The data are presented as ΔCt
values from three independent experiments. Student’s
independent samples t-test was used. (Abbreviations:
C: control healthy women; H-TS: exposed to TS without
COPD; ***P<0.0001).
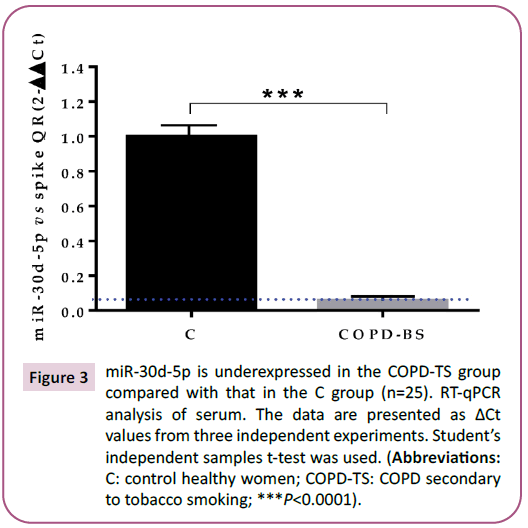
The two remaining miRNAs showed matching results with the
PCR array and RT-qPCR and were found to be underexpressed:
miR-30d-5p in the COPD-TS group compared with that in the C
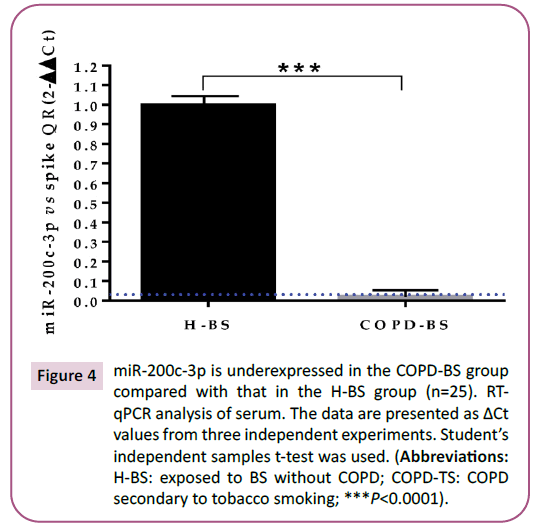
group (Figure 3; P< 0.0001); miR-200c-5p in women with COPDBS
compared with those with H-BS (Figure 4; P <0.0001).
Figure 3: miR-30d-5p is underexpressed in the COPD-TS group
compared with that in the C group (n=25). RT-qPCR
analysis of serum. The data are presented as ΔCt
values from three independent experiments. Student’s
independent samples t-test was used. (Abbreviations:
C: control healthy women; COPD-TS: COPD secondary
to tobacco smoking; ***P<0.0001).
Figure 4: miR-200c-3p is underexpressed in the COPD-BS group
compared with that in the H-BS group (n=25). RTqPCR
analysis of serum. The data are presented as ΔCt
values from three independent experiments. Student’s
independent samples t-test was used. (Abbreviations:
H-BS: exposed to BS without COPD; COPD-TS: COPD
secondary to tobacco smoking; ***P<0.0001).
Discussion
The PCR array study showed that 4 miRNAS were differentially
expressed a finding that was subsequently validated in RTqPCR
assays. Importantly, two of the five miRNAs that were
differentially expressed in the PCR array study did not coincide
with the results of the RT-qPCR, miR-10a-5p and miR-15b-5p,
that could be attributed, in part, to the genetic and epigenetic
susceptibility of each individual, socioeconomic and hereditary
family heterogeneity of the different women recruited, as well as
to the randomness in sample selection that was three women for
each group [13]. On the other hand, miR-30d-5p and miR-200c-
3p were coincident in both PCR array and RT-qPCR.
Based on the results of the RT-qPCR, miR-10a was overexpressed
in the COPD-TS compared with the H-TS group, miR-15b-5p
was underexpressed in H-TS related C group, miR-30d-5p was
underexpressed in the COPD-TS group compared with C, and
miR-200c-3p was underexpressed in the COPD-BS with H-BS
group. The deregulation of these serum miRNAs has not been
documented thus far, but especially with respect to women with
COPD-BS.
It is relevant to note that a search in various academic databases shows heterogeneity in the analysis performed in COPD, especially
since miRNAs have been examined in different body fluids such as
serum, plasma, BAL and sputum.
Concerning miR-10a-5p, has been shown that angiogenic
processes are inhibited when it miRNA is underexpressed in
rats exposed to TS [14]. However, in our validation between the
COPD-TS group regarding the H-TS, it was overexpressed. This
overexpression was observed in primary cultures of smooth
muscle cells of airways, where it inhibits the synthesis of DNA and
proliferation [15], that could be related to the pathogenic effects
that occur with the musculature in COPD, such is the case of
cachexia [1,2,5]. However, to corroborate this fact, it is necessary
to carry out focused studies to determine whether this miRNA
exerts this effect in women with COPD-TS.
miR-15b-5p was reported overexpressed in the lung tissue from
COPD-TS compared with healthy smokers, being its expression
different among patients with in different stages of COPD. The
expression of SMAD7 was validated as a target for miR-15b-5p,
being reduced in bronchial epithelial cells in COPD; instead, when
miR-15b-5p is underexpressed, there is an increase in SMAD7
protein inhibit apoptosis. However, we found that miR-15b-
5p was underexpressed in H-TS related to C group, supporting
the fact that miR-15b-5p could conferred a potential for the
development apoptosis in COPD-TS. However, this aspect needs
further clinical and experimental analysis [6].
miR-30d-5p that was underexpressed in women with COPD-TS
compared with the C group. It was analyzed mainly in the serum
of patients with nonsmall cell lung cancer (NSCLC), showing
a significant association between underexpression and longterm
patient survival in stages I-III; conversely, when it was
overexpressed, there was a lower survival [16,17], could serve as
a prognostic predictor of survival in NSCLC. In the case of women
with COPD-TS where it was underexpressed, it is necessary to
carry out further studies to determine whether it could have
diagnostic and prognostic values.
Regarding miR-200c-3p, is a regulators/inhibitors of the epithelialmesenchymal
transition, that maintenance the epithelial
phenotype in various cancers [18]. The downregulation of miR-
200c-3p induced by TS extract activates the nuclear factor NFKβ
pathway through the production of IL-6, contributing to the
mechanism of carcinogenesis [19]. miR-200c-3p also acts as an
inducer in the reduction of oxidative stress induced by H2O2 in
lung cell cultures [20]. Nevertheless, it is important to continue
studying this microRNA because, in the case of women exposed
to BS, it would seem to promote the development of COPD by
decreasing the antioxidant response, which requires a deeper
analysis.
Additionally, and to relevant, miR-22a-3p is of great interest
because it is underexpressed in women with COPD-BS
comparatively with those with COPD-TS, as was published
elsewhere [10]. miR-22a-3p has been use a biomarker in pulmonary
tuberculosis, NSCLC, and lung adenocarcinoma [21-23]; which,
it could also be a prognostic marker for the development of
COPD-BS, but this warrants further study. Additionally, serum
HDAC4, a target of miR-22a-3p, was incremented in COPD-BS
compared with COPD-TS [10,24].
Concerning miRNAs and HDAC4 in COPD-TS, these participate in
muscle dysfunction with and without mass loss, with impairment
and dysfunction of muscle strength in diaphragm and quadriceps
[25]. Furthermore, miR-22, when overexpression, induces a
strong response to Th17 cells and participates in the development
of pulmonary emphysema in smokers [24].
To contextualize the potential relevance of the four deregulated
miRNAs among the five groups of women in this study, also
considering miR-22-3b under-expressed in COPD-BS compared
to TS-COPD [22], we can comment that the overexpression of
miR-15-3b could help prevent COPD in smokers, keeping them as
healthy smokers; however, if miR-10a-5p overexpression occurs in these women, it could favor the development of COPD; on the
other hand, the under-expression of miR-30d-5p may contribute
to the development of emphysema more directly in female
smokers. In relation to the exposure to BS, the underexpression
of miR-200c-3p could contribute to the prevention of COPD,
maintaining the health of these women; while miR-22a-3p,
which was under-expressed compared to female smokers [10],
would participate more directly in the development of COPD.
Additionally, and taking in consideration that COPD by smoking is
considered a phenotype different to COPD by BS exposure, these
miRNAs, would be part of the molecular and pathophysiologic
mechanisms that operate specifically in each one of these
phenotypes.
Finally it is pertinent comment that this is the first comparative
study carried out in women with COPD-BS and COPD-TS (in
severe to very severe stages), healthy smokers, exposed to
BS without COPD, and healthy controls, demonstrating the
genotypic difference based on serum miRNA expression, which
a priori supports the fact that COPD from biomass has a different
phenotype from that of COPD from smoking, which could have
important implications for diagnosis, prognosis and treatment of
COPD [25].
Limitations
The limitations of this study are due, in the first place, to the
heterogeneity between the women of the different study groups,
which is relevant in terms of genetic susceptibility, ethnicity and
socioeconomic status, habitat and severity of exposure to BS or TS, which makes it difficult to determine the contribution of
each factor. Related to the validation of RT-qPCR, two miRNAs
overexpressed in the PCR matrix, contrary to the RT-qPCR, were
under-expressed, miR-10a-5b and miR-15b-5p, which would be
related to the small number in the sample (n = 3) and with the
randomness in the selection process. Regarding the size of the
sample used in the validation (n = 25), it is consistent with the
fact that the majority of women with COPD-TS are in GOLD IIIIV
status, which is not a limitation to select, while those with
COPD-BS commonly have GOLD I-II status, and rarely GOLD IIIIV
status, making it difficult to select a larger sample of COPDBS.
Additionally, it will be necessary in future protocols to
validate some of the target miRNAs from the mRNAs that were
deregulated between groups, to fully understand their possible
involvement in the of prevention or contribution in COPD.
Conclusion
Our findings suggest that women with severe to very severe BSCOPD
have a different serum miRNA expression profile compared
to those with TS-COPD, and with respect to healthy women
exposed to BS or TS; deregulated miRNAs could be potentially
relevant, miR-200c-3p, which was under-expressed in COPDBS,
while miR-10a-5p, which was overexpressed, and miR-15b-
5p and miR-30d-5p under-expressed in COPD-TS; nevertheless,
more studies are needed to corroborate the specific role of each
miRNA in the group of women that were compared, for which
the expression of some target mRNAs and their proteins might
be analyzed.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. (accessed 10 october 2020)
- Pérez-Padilla R, Ramirez-Venegas A, Sansores-Martinez R (2014) Clinical characteristics of patients with biomass smoke-associated COPD and chronic bronchitis, 2004-2014. Chronic Obstr Pulmonary Dis 1: 23-32.
- Camp PG, Ramirez-Venegas A, Sansores RH, Alva LF, McDougall JE, et al. (2014) COPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican women. Eur Respir J 43: 725-734.
- Rivera RM, Cosio MG, Ghezzo H, Salazar M, Perez-Padilla R (2008) Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis 12: 972-977.
- Barnes PJ (2016) Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 138: 16-27.
- Ezzie ME, Crawford M, Cho JH, Orellana R, Zhang S, et al. (2012) Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 67: 122-131.
- Chen X, Liang H, Zhang J, Zen K, Zhang CY (2012) Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol 22: 125-132.
- Weiland M, Gao XH, Zhou L, Mi QS (2012) Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol 9: 850-859.
- Velasco-Torres Y, Ruiz-López V, Pérez-Bautista O, Buendía-Roldan I, Ramírez-Venegas A, et al. (2019) miR-34a in serum is involved in mild-to-moderate COPD in women exposed to biomass smoke. BMC Pulm Med 19: 227
- Velasco-Torres Y, Ruiz V, Montaño M, Pérez-Padilla R, Falfán-Valencia R, et al. (2019) Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass. Biomolecules 9: 837.
- Pérez-Padilla JR, Regalado-Pineda J, Vázquez-García JC (2001) Reproducibility of spirometry in Mexican workers and international reference values. Salud Publica Mex 1: 113–121.
- Marabita F, De Candia P, Torri A, Tegner J, Abrignani S, et al. (2012) Normalization of circulating microRNA expression data obtained by quantitative real-time RT-PCR. Brief Bioinform 17: 204-212.
- Sandford AJ, Chagani T, Weir TD, Connett JE, Anthonisen NR, et al. (2001) Susceptibility genes for rapid decline of lung function in the lung health study. Am J Respir Crit Care Med 163: 469-473.
- Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S (2009) Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 23: 806-812.
- Hu R, Pan W, Fedulov AV, Jones MR, Weiss ST, et al. (2014) MicroRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway. FASEB J 28: 2347-2357.
- Hu Z, Chen X, Zhao Y, Tian T, Jin G, et al. (2010) Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 28: 1721-1726.
- Roth C, Stuckrath I, Pantel K, Izbicki JR, Tachezy M, et al. (2012) Low levels of cell-free circulating miR-361-3p and miR-625* as blood-based markers for discriminating malignant from benign lung tumors. PLoS One 7: e38248.
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, et al. (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 68: 7846-7854.
- Zhao Y, Xu Y, Li Y, Xu W, Luo F, et al. (2013) NF-kappa B-mediated inflammation leading to EMT via miR-200c is involved in cell transformation induced by cigarette smoke extract. Toxicol Sci 135: 265-276.
- Wu YH, Lin HR, Lee YH, Huang PH, Wei HC, et al. (2017) A novel fine tuning scheme of miR-200c in modulating lung cell redox homeostasis. Free Radic Res 51: 591-603.
- Zhang X Guo J, Fan S, Li Y, Wei L, Yang X, et al. (2013) Screening and identification of six serum microRNAs as novel potential combination biomarkers for pulmonary tuberculosis diagnosis. PLoS One 8: e81076.
- Xu C, Zheng Y, Lian D, Ye S, Yang J, et al. (2015) Analysis of microRNA expression profile identifies novel biomarkers for non-small cell lung cancer. Tumori J 101: 104-110.
- Dong HX, Wang R, Jin XY, Pan J (2018) LncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via inhibiting hsa-mir-22-3p. J Cell Physiol 233: 4126-4136.
- Lu W, You R, Yuan X, Samuel ELG, Marcano DC,et al. (2015) The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat Immunol 16:1185-1194.
- Puig-Vilanova E, Aguiló R, Rodríguez-Fuster A, Martínez-Llorens J, Gea J, et al. (2014) Epigenetic mechanisms in respiratory muscle dysfunction of patients with chronic obstructive pulmonary disease. PLoS One 9: e111514.