Research Article - (2022) Volume 8, Issue 3
Deregulation of Plasma MiR-21 for Early Detection of Lung Cancer among Palestinian People Exposed to High Intensity of Diesel Exhaust Emissions
Ahmed Isam Slaileh*,
Ashraf Sawafta and
Raed Alkowni
Department of Molecular Biology, An-Najah National University, Nablus, Palestinian Territory
*Correspondence:
Ahmed Isam Slaileh, Department of Molecular Biology, An-Najah National University, Nablus,
Palestinian Territory,
Tel: 970599650132,
Email:
Received: 04-Sep-2022, Manuscript No. IPBMBJ-22-14221;
Editor assigned: 07-Sep-2022, Pre QC No. IPBMBJ-22-14221 (PQ);
Reviewed: 22-Sep-2022, QC No. IPBMBJ-22-14221;
Revised: 10-Mar-2022, Manuscript No. IPBMBJ-22-14221 (R);
Published:
22-Mar-2022, DOI: 10.36648/2471-8084-9.1.111
Abstract
For many years, intensive research on the early detection of lung cancer has been of interest to many scientists. Many studies recently focused on using microRNAs as an effective plasma biomarker for the diagnosis of different types of cancer including lung cancer. MIR-21 deregulation was observed in lung cancer patients and plays an important role in tumor development. In this present study, we investigated the plasma miR-21 biomarker as a non-invasive and cost-effective test for early detection of lung cancer among people working at a transportation center in Palestine since these people are exposed to a high level of Diesel Motor Emission (DME). Real time PCR revealed a significant increasing in the expression level of miR-21 in human plasma samples exposed to DME and in Non Small Cell Lung Cancer (NSCLC), than that of normal samples. Moreover, our results indicate a significant correlation between the quantity of plasma miR-21 and the exposure duration to DME (p˂0.05). These results confirm the significance of using miR-21 as a biomarker for the early detection of lung cancer among high risk people.
Keywords
Plasma microRNA; MiR-21; Lung cancer; Diesel exhaust emissions; Biomarkers; Real time
PCR
Introduction
Lung cancer is a global problem and the most common type
of cancer causing death. The mortality rate of lung cancer in
palestine is highest among males than other tumors [1]. The
prevention and treatment of lung cancer are successfully
achieved when it was early detected. Moreover, 60%-80%
survival at 5 years is investigated in patients diagnosed with
early stage disease. Lung cancer is closely related to chemical
and environmental factors which lead to genetic instability
and tumor development. Because the majority of lung cancer
patients are diagnosed with stage III or VI, novel plasma biomarkers for early detection of lung cancer are needed
since they are simple, noninvasive and reliable diagnostic test
[2].
Diesel Motor Emissions (DME) or diesel exhaust are a complex
mixture of particles and gases containing hundreds of
chemical compounds. Diesel exhaust particles are composed
of a center core of elemental carbon and adsorbed organic
compounds, in addition to a few amounts of nitrate, sulfate
and metals. While nitrogen compounds, carbon monoxide,
sulfur compounds and many hydrocarbons compounds such
as benzene, aldehydes, Polycyclic Aromatic Hydrocarbons (PAHs) and nitro PAHs are classified as gaseous components
[3]. DME contains highly mutagenic and
carcinogenic particles, especially nitrous oxides and Polycyclic
Aromatic Hydrocarbons (PAH).
MicroRNAs (miRNAs) are a family of non-coding small RNAs,
which consist of 19 to 25 nucleotides and have main roles in
the regulation of gene expression at the post-transcriptional
level by either degradation of mRNAs through base pairing
to the Untranslated Region (UTR) of the mRNAs molecule or
by inhibition the translation process through preventing the
binding of the ribosome to mRNAs molecule [4]. Furthermore,
it has been suggested that miRNAs target and regulate
about 30% of human genes involved in various biological
functions, including cellular proliferation, cell death, fat
metabolism and differentiation.
Many studies have proven that miRNA expression
alterations occur not only in many human diseases but also
in cancer. In addition, deregulation of miRNA expression was
observed in tumor tissues with variations according to tumor
type, compared with normal tissues [5]. miRNAs play an
important role in tumor suppression, which
contributes to the development of a malignant cell when
loses its function by mutations and genomic alterations.
MiR-21
MiR-21 is 22 nucleotides located at 17 q23.1. It is classified
as an oncogene and considered as anti-apoptotic factor.
Over-expression of miR-21 minimizes the expression of
apoptotic genes [6]. The over expression of miR-21 not only
observed in lung cancer but also in breast cancer, colon
cancer, liver and brain cancers. Recent studies on miR-21
were shown that it affects and suppresses four tumor
suppressor genes including mapsin, Phosphatase and
Tensin homolog (PTEN), Tropomyosin1 (TPM1) and
Programmed Cell Death 4 (PDCD4). MiR-21 prevents the
translation of these four genes by binding to their UTR of the
transcript. The results are tumor growth, cell transformation
and metastasis [7]. In this study, we evaluated the plasma
miR-21 expression levels in people exposed to DME,
patients with lung cancer and healthy individuals to validate
it as an acceptable biomarker for early detection of lung
cancer.
Materials and Methods
Study Population
The study population was 40 samples; divided into three
groups, the first one is composed of 20 samples from people
working at the downtown transportation center in Nablus for at least 4 hours of daily exposure to diesel exhaust
emissions for a long time. The second group is consist of 10
samples from healthy individuals (cancer free control) and
the final group is 10 samples of non-small cell lung cancer
patients who are under treatment at An-Najah National
University Hospital [8]. All samples were nonsmokers
and healthy controls were free of cancer history.
Plasma Preparation
5 ml of peripheral blood samples were collected from all DME
and healthy individuals at a private lab, while lung cancer
control samples were collected at An-Najah university
hospital. Clinical information was collected from each
individual using a questionnaire [9]. Fresh whole blood
samples in EDTA preservative were centrifuged immediately
at 1900 g on 4°C for 10 minutes. Plasma was transferred to a
new RNase-free tube. Plasma samples were centrifuged on
1600 g at room temperature for 10 minutes and then
supernatants were transferred to new RNase free tubes and
stored at -80°C until use.
Plasma RNA Extraction
Total RNA containing small RNA was extracted from 200 μl of
plasma using miRNeasy serum/plasma kit (Qiagen) according
to the manufacturer’s protocol. The final elution volume was
12 μl in RNase-free water. The concentration and purity of
RNA samples were determined by NanoDrop 1000 (Thermo
Fisher Scientific) [10].
Poly (A) Tailing of MiRNAs and cDNA Synthesis
25 μl of total RNA sample containing miRNA was
polyadenylated using poly (A) polymerase (PAP, Ambion) and
then reversely transcribed to cDNA using modified MMLV
ultra script reverse transcriptase (PCR Biosystems) according
to the manufacturer’s instructions with a poly (T) adapter
primer (5’GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTT
TTTTTT TTVN-3’) (Shi and Chiang, 2005).
MicroRNA Quanti ication by Real Time RT-PCR
SYBR Green based quantitative real time PCR assay was
performed for miRNA expression quantification using 2 ×
qPCRBIO SyGreen Blue Mix Hi-ROX (PCR Biosystems) with
specific forward primer and universal reverse primer which
were complementary sequence to poly (T) adapter primers in
Bio-Rad CFX Maestro system. The PCR was performed in
duplicate as following steps: initial denaturation at 95°C for 2
minutes, then 50 cycles of 95°C for 5 seconds and 60°C for 30
seconds (Table 1).
| Primer |
Sequence |
| Universal reverse primer |
5’-GCGAGCACAGAATTAATACGACTCA -3’ |
| miR-21 (forward) |
5’-TAGCTTATCAGACTGATGTTGA -3’ |
Table 1: The sequences of primers.
Generation of the Standard Curve for Absolute
Quantification of MiRNAs
Synthetic single stranded miRNA as an internal control for
miRNA expression profiling (miRNeasy serum/plasma spike-in
control) was purchased from Qiagen. Synthetic spike-in
miRNA (Ce_miR-39) was polyadenylated and reverse
transcribed to cDNA according to the manufacturer’s protocol
in order to generate a standard curve for the miRNA SYBR PCR
assays [11]. The assessment of RNA recovery was achieved by
comparing CT value to a synthetic miRNA standard curve
generated independently of the RNA extraction. The absolute
concentration of spike-in control was 1 x 106 copies/μl. Serial
dilutions of spike-in control cDNA were prepared for standard
curve generation for estimation of the recovery of spike-in
control which was added to the plasma samples.
Results
Sensitivity, Specificity, PPV and NPV of MiRNA
Quantification by Real-Time PCR
95% of DME exposed samples show over expression of
miR-21 in comparison to spike-in control [12]. No
amplification occurs in NTC (no Ct value) as evidence of the
absence of any contamination and primer dimer
amplification. Also, all lung cancer patients’ samples show an
elevation in the expression of miR-21 compared with spike-in
control. While 90% of these samples show over expression
compared with healthy control samples with 95% sensitivity
and 60% specificity, while PPV and NPV were 82.6% and
85.7% respectively (Figure 1).
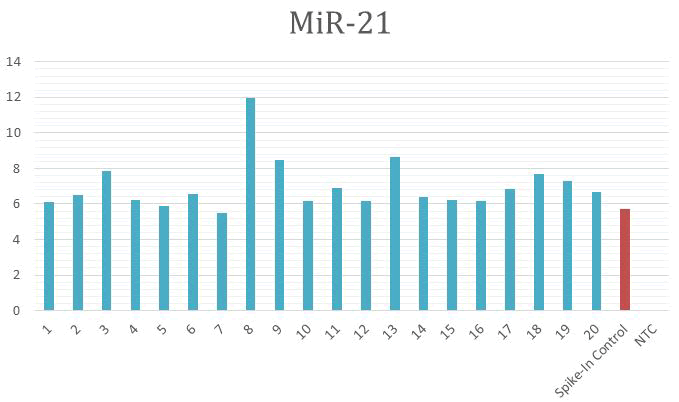
Figure 1: MiR-21 expression of all DME exposed samples (1 to
20), spike-in control and Non-Template Control (NTC) using
qPCR.
The comparison between the expression of miR-21 and
Cel_miR-39 spike-in control in all samples including DME
exposed samples, healthy control and lung cancer patients
control were shown in the above figures. Nineteen samples
out of twenty DME exposed samples showed over expression
of miR-21 (95% of all DME exposed samples) [13]. While 100%
of lung cancer patients control samples and 40% of healthy
controls showed over-expression of plasma miR-21 (Figure 2).
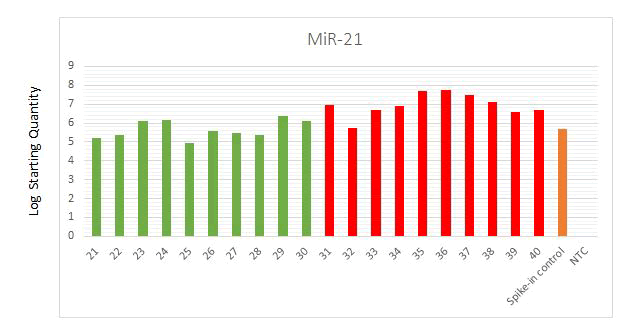
Figure 2: MiR-21 expression of healthy samples (21 to
30), lung cancer patients (31 to 40), spike-in control and
Non Template Control (NTC) using qPCR.
Identification of Lung Cancer Biomarker for High Risk
People
The comparison between all groups in the quantity of plasma
miR-21 is shown in the above figure [14,15]. DME exposed
samples have a higher mean (7.01, SD=1.44), while the
healthy controls have a lower (5.66, SD=0.48), while the mean
for lung cancer controls was (6.96, SD=0.58). Based on the
significant difference in means between these three groups
(ANOVA, F=4.552, P˂0.05), healthy controls are responsible
for the significant difference compared with DME exposed
people and lung cancer patients based on post hoc
comparison (Figure 3).
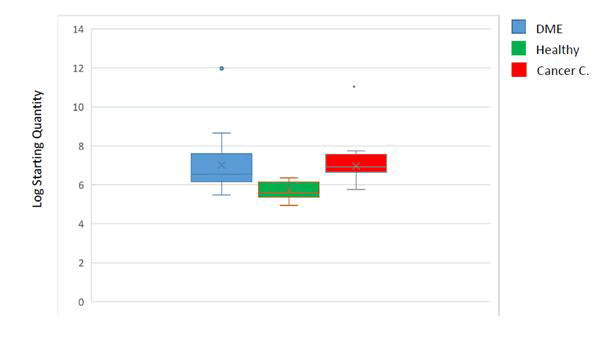
Figure 3: Box plot shows the correlation between plasma
levels of MIR-21 in healthy control (cancer-free control), DMEexposed
people, and lung cancer patients. The line inside the
boxes denotes the medians. The boxes mark the interval
between the 25th and 75th percentile. The Whiskers denote
the interval between the 5th and 95th percentiles.
Efforts are focusing on the early detection of cancer including
lung cancer to reduce the mortality [16,17]. Human cancer
exhibits an alteration in the miRNAs profile with tumor
suppressive and oncogenic activity [18]. MiRNAs proved their
ability in the early detection of lung cancer and are
considered a biomarker depending on many studies in this
field. Many recent studies investigated the relationship
between the upregulation of miR-21 and different human
cancers. It has been reported that miR-21 suppresses PTEN
which can induce apoptosis, control cell growth and
angiogenesis. The expression of miRNAs varies in plasma
samples of high cancer risk people who are exposed to different carcinogens including DME. In our study, we focused
on the plasma levels of miR-21 in people who work at a high
intensity of DME which may be related to lung tumorigenesis
[19]
In our results, 95% of DME exposed samples show over
expression of miR-21 [20] Moreover, 40% of healthy controls
and 100% of lung cancer patients’ samples control show over expression of plasma miR-21 level (Table 2). However,
miR-21 is not a specific biomarker for lung cancer only, but
also for different types of cancer including breast cancer,
colon cancer, hepatocellular carcinoma and glioblastoma [21].
| |
Healthy C. |
DME exposed |
Cancer C. |
| M (SD) |
M (SD) |
M (SD) |
| Log starting quantity of miR-21 |
5.66 (0.48) |
7.01 (1.44) |
6.96 (0.58) |
| Age |
32.80 (13.08) |
45.90 (12.06) |
60.2 (4.62) |
| No. of years in the transportation center |
0 |
10.50 (2.87) |
N/A |
| Exposure duration (hrs /day) |
0 |
3.60 (0.681) |
N/A |
| Total Exposure duration (hrs) |
0 |
11918.40 (4411.88) |
N/A |
| miR-21 expression |
n (%) |
n (%) |
n (%) |
| Normal regulation |
6 (60%) |
1 (5%) |
0 (0%) |
| Up regulation |
4 (40%) |
19 (95%) |
10 (100%) |
| Smoking history |
0 (0%) |
0 (0%) |
8 (80%) |
| Male |
8 (80%) |
20 (100%) |
10 (100%) |
| Adenocarcinoma |
N/A |
N/A |
8 (80%) |
| Squamous cell carcinoma |
N/A |
N/A |
2 (20%) |
Table 2: Demographic and histopathologic data for plasma samples.
The expression of miR-21 in DME exposed samples 8 and 13
was the most with a starting quantity of 7466.18 × 105 and
4518.5 × 105 copies/μl respectively. These results indicate the
high exposure intensity of DME for these people working at
the transportation center which is about 6 hours daily for 15
years depending on the participants’ history and questioners
[22].
Although all lung cancer patients participating in this research
are under treatment at An-Najah National University Hospital,
the results show a significant elevation in the expression of
miR-21 compared with healthy and spike-in control with 95%
sensitivity. The relatively low false negative results mean the
ability to identify most of lung cancer patients with a disease.
The high sensitivity and relatively high specificity indicate that
they could discriminate plasma samples of DME exposed from
healthy controls and they will be a novel noninvasive
biomarker for early detection of non-small cell lung cancer.
Recently, Shen concluded that the plasma level of many
miRNAs including miR-21 is useful for discriminating healthy
controls from Non-Small Cell Lung Cancer (NSCLC) patients
with 86.22% sensitivity and 96.55% specificity, which supports
the hypothesis that miRNAs may use as novel biomarkers for
early detection of lung cancer.
Discussion
In Our results, there is a statistical significance difference
between three groups (healthy control, DME exposed and
lung cancer patients control) and the number of miRNAs in
plasma samples. Importantly, miR-21 was significantly over
expressed in DME exposed people and lung cancer patients
compared with healthy control. This study provides a shared
of evidence that occupational exposure to DME correlates
with elevation of the expression of miR-21, which is
considered a biomarker for high risk of lung cancer depending
on the results that showed an odds ratio of OR=28.5 (95% CI
2.6-306). On another hand, high NPV obtained from results
using real time PCR for quantification of plasma miR-21 of
high risk people with high-intensity exposure to DME
indicates the ability to depend on this screening for early
detection and there is less need for other expensive and
invasive tests such as chest biopsy and chest CT.
Conclusion
There is a great need to develop early detection screening
tests for lung cancer since the rate of mortality is increased
when the disease is diagnosed in its later stages. In
conclusion, our study strengthens the arguments which report plasma miR-21 could be a potential non-invasive and
cost effective biomarker for early detection and diagnosis of
lung cancer. Due to the few reports on the plasma biomarkers
for early detection of different cancer types in palestine, the
results of this study have confirmed the significance of using
miRNAs in the early detection of cancer. Many further studies
on different miRNAs such as miR-155, miR-15a, miR-15b,
miR-126, miR-127 and miR-197 which are implicated in
human lung cancer are needed.
Acknowledgment
We thank Mahmoud Ruzaygat for his consultation in qPCR
and Dr. Jamal Qaddumi for his assistance in statistical analysis.
This study was mainly supported by An-Najah National
University and An-Najah University Hospital.
References
- Bianchi F, Nicassio F, Marzi M, Belloni E, Dall'Olio V, et al. (2011) A serum circulating miRNA diagnostic test to identify asymptomatic high‐risk individuals with early stage lung cancer. EMBO Mol Med. 3(8):495-503.
[Crossref] [Googlescholar]
- Gatsonis CA, Aberle DR, Berg CD, Black WC, Church TR, et al. (2011) The national lung screening trial: Overview and study design. Radiology. 258(1):243-253.
[Crossref] [Googlescholar] [PubMed]
- Wichmann HE (2007) Diesel exhaust particles. Inhalation Toxicol. 19(1):241-244.
[Crossref] [Googlescholar] [Indexed]
- Pederson TC, Siak JS (1981) The role of nitroaromatic compounds in the direct acting mutagenicity of diesel particle extracts. J Appl Toxicol. 1(2):54-60.
[Crossref] [Googlescholar] [PubMed]
- Steenland K (1986) Lung cancer and diesel exhaust: A review. Am J Ind Med. 10(2):177-189.
[Crossref] [Google Scholar] [PubMed]
- Garzon R, Calin GA, Croce CM (2009) MicroRNAs in cancer. Annu Rev Med. 60:167-179.
[Crossref] [Google Scholar] [PubMed]
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ (2004) Processing of primary microRNAs by the microprocessor complex. Nature. 5(432):231-235.
[Crossref] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, et al. (2004) The microprocessor complex mediates the genesis of microRNAs. Nature. 432(7014):235-240.
[Crossref] [Google Scholar] [PubMed]
- Diederichs S, Haber DA (2007) Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 131(6):1097-1108.
[Crossref] [Google Scholar] [PubMed]
- Shenouda Sk, Alahari SK (2009) Microrna Function In Cancer: Oncogene Or A Tumor Suppressor? Cancer Metastasis Rev. 28(3):369-378.
[Crossref] [Googlescholar] [PubMed]
- Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 65(14):6029-6033.
[Crossref] [Google Scholar] [PubMed]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, et al. (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 103(7):2257-2261.
[Crossref] [Google Scholar] [PubMed]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, et al. (2008) MicroRNA-21 (miR-21) post transcriptionally downregulates tumor suppressor PDCD4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 27(15):2128-2136.
[Crossref] [Google Scholar] [PubMed]
- Zhu S, Wu H, Wu F, Nie D, Sheng S, et al. (2008) MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 18(3):350-359.
[Crossref] [Google Scholar] [PubMed]
- Zhu S, Si ML, Wu H, Mo YY (2007) MicroRNA-21 targets the tumor suppressor gene Tropomyosin 1 (TPM1). J Biol Chem. 282(19):14328-14336.
[Crossref] [Googlescholar] [PubMed]
- Shi R, Chiang VL (2005) Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 39(4):519-525.
[Crossref] [Googlescholar] [PubMed]
- Zheng D, Haddadin S, Wang Y, Gu LQ, Perry MC, et al. (2011) Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol. 4(6):575.
[Google Scholar] [PubMed]
- Shen J, Todd NW, Zhang H, Yu L, Lingxiao X, et al. (2011) Plasma microRNAs as potential biomarkers for non small cell lung cancer. Laboratory Investigation. 91(4):579-587.
[Crossref] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, et al. (2003) Redox regulation of PI 3‐kinase signaling via inactivation of PTEN. EMBO J. 22(20):5501-5510.
[Crossref] [Google Scholar] [PubMed]
- Stewart AL, Mhashilkar AM, Yang XH, Ekmekcioglu S, Saito Y, et al. (2002) PI3K blockade by Ad-PTEN inhibits invasion and induces apoptosis in radial growth phase and metastatic melanoma cells. Mol Med. 8(8):451-461.
[Google Scholar] [PubMed]
- Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, et al. (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 9(3):189-198.
[Crossref] [Google Scholar] [PubMed]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, et al. (2007) MiR-21 mediated tumor growth. Oncogene. 26(19):2799-2803.
[Crossref] [Google Scholar]
Citation: Slaileh AI, Sawafta A, Alkowni R (2023) Deregulation of Plasma Mir-21 for Early Detection of Lung Cancer
among Palestinian People Exposed To High-Intensity of Diesel Exhaust Emissions. Biochem Mol Biol J. 9:111.
Copyright: © 2022 Slaileh AI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution
License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source
are credited.




