Introduction
In the last decade, there has been a phenomenal advancement
in theoretical inorganic chemistry [1,2], much faster computers
are available and commercial programs incorporating the latest
methods have become widely available and are capable of
providing more information about molecular orbitals (MOs), with
a sample input of chemical formula. The focus of attention has
been on computational transition-metal chemistry [3,4]. This is
largely due to the successful employment of gradient corrected
density functional theory in calculating molecules, particularly of
the heavier atoms [5-8] and in the use of small-core relativistic
effective core potential [9-11] which set the stage for calculation
of geometries, bond energies, and chemical reaction and other
important properties of transition metal compounds with
impressive accuracy [8,12]. Application of density functional
calculation to organometallic [13,14] and transition metal compounds is growing [15] density functional parameters
such as eigenvectors, eigenvalues and population analysis are
well calculated with this method. In this paper present the
calculations of Eigen vectors, Eigen values and population analysis
of cobaltocene and nickelocene in order to study the extent of
contribution of 3d, 4s and 4p orbital in the formation of MOs.
Such a quantitative study will provide correct information about
the involvement of 4p orbital of cobalt and nickel in bonding will
help to resolve the controversy raised by other workers [16-20].
Materials and Methods
In computational chemistry tools the DFT offers the fundamentals
for interpreting multiple chemical concepts used in different
branches of chemistry. In modern computational chemistry,
quantum chemical calculations are typically performed with in
a finite set of basic functions. When molecular calculations are performed, it is common to use a basis sets composed of a finite
number of atomic orbitals, centered at each atomic nucleus
with in the molecule, for example linear combination of atomic
orbitals. The methods most commonly used for this research are
DFT/B3LYP
1. combination of Beck’s three-parameter exchange functional
and Lee-Yang-Parr correlation functional with 6-31G
2. basis set.
These methods are found in Gaussian 03W program. B3LYP is
a DFT method with hybrid functional that provides qualitative
results at a lower cost than abinitio methods with a comparable
accuracy [21]. By using these methods we have optimized the energy, Eigen values, Eigen vector, population analysis, HOMOLUMO
energy gap, hardness, softness, electronegativity, visualize
the HOMO and LUMO orbitals’ of cobaltocene and nickelocene
molecules. The coefficients in linear combination for each
molecular orbital being found by solution of the Roothaan-equation.
A widely used method to analyze SCF wave function is population
analysis, introduced by Mullikan population methods [22].
Results and Discussion
This research is aimed to study the electronic structure and
optimized geometry of cobaltocene and nickelocene molecules.
Geometry optimization is used to find minima on the potential
energy surface representing equilibrium structure and used to
obtain structure for a single-point quantum mechanical calculation, which provides a large set of structural and electronic
properties. The electronic structure and geometry of cobaltocene
and nickelocene molecules are found through DFT/B3LYP with a
basis set of 6-31G (d) calculations. The optimized structures of
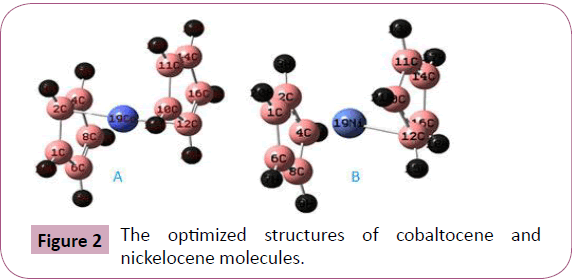
these two compounds are shown in Figure 1A and 1B respectively
for cobaltocene and nickelocene. The significant computed
parameters are available in Tables 1 and 2 including the bond
lengths, bond angles and dihedral angles of these two compounds.
The optimized bond length of C-C double and single bonds in
cobaltocene rings fall 1.36-1.96˚A, and nickelocene 1.392-1.98˚A
at DFT/B3LYP, levels through 6-31G (d) basis set. There are two
types of C-C bonds involved in these species. These are C-C single
bonds and C-C double bonds of cobaltocene and nickelocene and
according to its bond length are in the order of C=C<C-C. From Tables 1 and 2 we observed slight difference in the bond lengths,
bond angles and dihedral angles throughout the molecules of
ferrocene and nickelocene. This indicates that the aromatic cobalt
atom in cobaltocene and nickel atom in nickelocene are relatively stable metabolically. As shown in Figure 2A and Table 1 the bond
connectivity of Co-(C5H5)2 of the two ligands are asymmetrical.
The optimized bond length of cobalt atom with the two carbon
atoms has fewer variations. The cobalt atom in cobaltocene is
bonded with C2 atom of bond length 1.969 (˚A) in one side of the
ligand and with C12 of bond length 1.994 (˚A) on the opposite
side. In the cobaltocene molecule the cobalt atom is located
between the two ligands but inclined by -80.623 measured from
the plane of the cyclopentadienyl and the two ligands are parallel
but with a slide of one from the other by a center of mass
separation 1.33˚A. As shown in Figure 2B and Table 2. The bond
connectivity of Ni-(C5H5)2 of the two ligands are asymmetrical.
The nickel atom in nickelocene is bonded with C12 atom of bond
length 1.976 (˚A) only from one side of the ligand. This is due to
the weak ligand fields of nickelocene having high spin arrangement
with two d electrons and low spin arrangement with six d
electrons of nickel atom which resulted in more reactivity of
nickelocene molecule with respect to the other two molecules. In
the nickelocene molecule the nickel atom is located between the
two ligands but inclined by -67.377o as measured from the plane
of the cyclopentadiene and the two ligands are almost parallel
but with a slide of one from the other by a center of mass
separation of only 0.22˚A. Generally comparing the bond length
and bond angles between metal atom and carbon in cobaltocene
and nickelocene molecules the former molecule possesses higher
bond angles and the later molecule possesses larger bond length.
The large the bond length the less stability but more reactivity,
hence nickelocene is more reactive and less stable than the
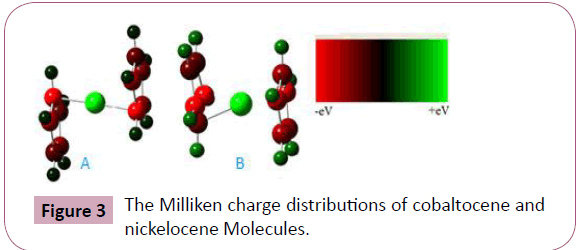
cobaltocene. In the calculations of Mullikan charge distributions
of cobaltocene and nickelocene molecules, given in Figure 3. The
red color indicates for excess of negative charges (-ve) while the
green color indicates for excess of positive charges (+ve) among
the bonded atoms, where electrons can flow from positions of
excess of negative charges (-ve) to the positions of excess of
positive charges (+ve). Energies of molecular orbitals are called
Eigen values. The main focus has been on the molecular structure and the properties that will be evaluated can be used to determine
the molecular reactivity as well as the molecular stability. The
HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest
Unoccupied Molecular Orbital) are very important aspects to
consider for these types of observations. This is because the
HOMO and LUMO are the most likely locations where reaction
will occur. The reaction is likely to occur there because the
electrons in the HOMO have the highest energy and therefore
the electrons are most willing to react. The LUMO is likely the
location for a bond to occur as well because any invading electrons
for another molecules will fill in to the LUMO, that is why
comparing the energies of these orbitals create an idea of how
reactive a molecule is important parametric properties of the
molecules at the DFT/B3LYP levels in 6-31G (d) basis set has been
calculated and are given in Table 3. At the DFT/B3LYP level the
HOMO energy of cobaltocene is -0.6452 eV which is slightly more
negative than nickelocene of -0.6427 eV and the LUMO energy of
cobaltocene is -0.5626 eV, and nickelocene -0.56142 eV. The
HOMO-LUMO gap of cobaltocene and nickelocene are 0.0826
and 0.0813 eV respectively. These proves that the positions of
HOMO, LUMO and the HOMO-LUMO gap can predict the stability
and reactivity of the molecules, and the cobaltocene molecule
shows relatively high energy gap value and the data here
suggested that cobaltocene is relatively less reactive and more
stable than nickelocene molecule. The most stable MO energy of
cobaltocene and nickelocene are respectively -277.5151 -eV, and
-295.6703 -eV. In general the HOMO and LUMO energy gap
reveals the chemical activity of the molecules. LUMO as an
electron acceptor represents the ability to obtain an electron (i.e. the electron affinity) and HOMO as an electron donor represents
the ability to donate an electron from its orbital (i.e. the Ionization
Potential). The less values in the HOMO-LUMO energy gap
explains eventually charge transfer interaction taking place within
the molecules. Hard molecules have large HOMO-LUMO energy
gaps and soft molecule have small HOMO-LUMO energy gaps. So
soft molecules (molecules with small energy gap) are favorable
for easy reaction. This description also supports for cobaltocene
and nickelocene molecule, cobaltocene is harder than
nickelocene. In Table 3 the HOMO-LUMO gap, as a characteristic of reactivity, shows cobaltocene has lower chemical reactivity
comparing to nickelocene molecule. Absolute hardness and
softness are important properties to measure the molecular
stability and reactivity. It is apparent that the chemical hardness
fundamentally signifies the resistance towards the deformation
or polarization of the electron cloud of the atoms, ions or
molecules under small perturbation of chemical reaction. A hard
molecule has a large energy gap and a soft molecule has a small
energy gap. So for more energetically stable and less reactive
cobaltocene molecule, the HOMO-LUMO energy gap and
hardness, η is larger comparing to nickelocene molecules. The
dipole moments and Mulliken charge ranges as displayed in Table 3. Nickelocene would have more charge than the
cobaltocene molecule. This is due to higher dipole moment and
lower HOMO-LUMO energy gap indicated that the molecule is
better reactive. This indicates that nickeloceneis more polar so
that it will react with polar solvents like water. Since the separation
between mass centers of the two ligands is small. The higher the
dipole moment, the more polar a molecule is. This could mean
that the receptor is more likely to accept polar molecules into its
active site. The receptor’s active sites may serve as home to
atoms that have very high electron affinities that attract the
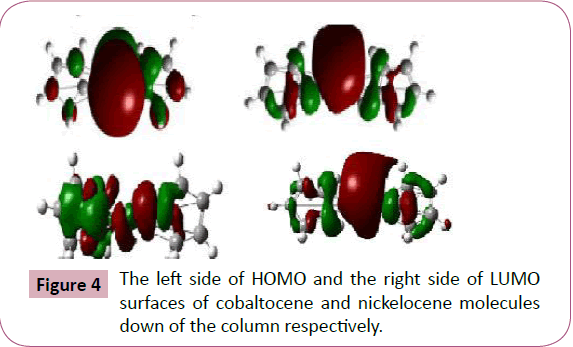
negatively charged end of a polar molecule. The above figures
shows the visualized structures of cobaltocene and nickelocene
show the population of electrons on their orbitals. The HOMO
Orbitals represented by green color, whereas for LUMO, it is
represented by red color. The red color represents the negatively
charged areas of surface (i.e. those areas where accepting the
electrophiles is most favorable) while the green color represents
the positively charged areas of surface (i.e. those areas where
accepting the nucleophiles is more favorable). The electron
density of HOMO and LUMO of cobaltocene and nickelocene
molecule are concentrated throughout the compound except at
the right and left terminals where some of the π∗ orbitals may be
empty. Eigen vector values of atomic orbitals have been evaluated
for the study of linear combination of atomic orbitals (LCAO). The
MOs of cobaltocene and nickelocene are formed by linear
combination of fifty AOs of two (C5H5)- and nine orbital of cobalt
and nickel. These fifty-nine AOsχ1 to χ59 on LCAO approximation
form same number of MOs, Φ1 to Φ59. The AOs χ1 to χ40 for 2s,
2px, 2py, 2pz of 1C to 10C, χ41 to χ49 for 4s, 4px, 4py, 4pz, 3dx2-y2,
3d2z, 3dxy, 3dxz, 3dyz of 11M and χ50 to χ59 for 1s of 12H to 21H
respectively, where M=Co and Ni, for cobaltocene and
nickelocene, respectively. The 2s, 2px and 2py orbitals of each
carbon atom of (C5H5)- are involved in the formation of σ bond
between C-C and C-H. The orbitals involved in σ bond hence shall
remain out of discussion. The 2pz orbitals of ten carbons and nine
orbitals of cobalt or nickel i.e. in total nineteen orbitals are
relevant to our discussion in respect of bonding between cobalt
or nickel orbitals and 2pz orbital of (C5H5)-. These atomic orbitals
are χ4, χ8, χ12, χ16, χ20, χ24, χ28, χ32, χ36 and χ40 of carbon and
χ41 to χ49 of cobalt and nickel. The coefficients of these orbitals
are the eigenvector values of χ [21].They express the forms of
MOs i.e. the extent of involvement of χ in the formation of Φ. In
order to examine the contribution of various atomic orbitals in
the formation of molecular orbitals the Eigen vector analysis has
been made and studied and data are given in Tables 4-11 respectively. The coefficients of these orbital are the Eigen vector values of, χ which have been evaluated by density functional
method using Gaussian-03 software. They express the form of
molecular orbital that is the extent of involvement of χ in the
formation of Φ. The calculated Eigen vector values of atomic
orbitals of Co and Ni in the formation of molecular orbitals in
cobaltocene and nickelocene in Tables 4, 5, 8, 9 respectively and
the calculated Eigen vector values of 2pz orbital of carbon are
given in Tables 6, 7, 10, 11. Tables 5, 7, 9, 11 are summation of
Eigen vector values of cobaltocene and nickelocene. Negative,
Zero and near zero coefficient values are negligible contributions
[21,23] of electrons and have been excluded from the Tables
(Figure 4).
| Entry |
Bond |
Entry |
Bond |
Entry |
Dihedral |
| |
length(˚A) |
|
angle (o) |
|
angle (o) |
| C1-C2 |
1.509 |
C6-C1-C2 |
109.511 |
C6-C1-C2-C4 |
0.027 |
| C1-C6 |
1.509 |
C8-C6-C1 |
109.511 |
C8-C6-C1-C4 |
-0.028 |
| C8-C6 |
1.359 |
C10-C11-C1 |
109.511 |
C10-C11-C14-C16 |
-78.168 |
| C10-C11 |
1.509 |
C10-C12-C1 |
109.56 |
C16-C12-C10-C11 |
84.623 |
| C10-C12 |
1.509 |
Co-C2-C1 |
79.123 |
Co-C2-C1-C6 |
-80.623 |
| C11-C14 |
1.909 |
|
|
|
|
| C12-C16 |
1.559 |
|
|
|
|
| C2-C4 |
1.359 |
|
|
|
|
| C4-C8 |
1.87 |
|
|
|
|
Table 1: The selected bond lengths in °A, some bond angles and dihedral angles of the optimized structure of cobaltocene using DFT levels with B3LYP/6-31G (d) basis set.
| Entry |
Bond |
Entry |
Bond |
Entry |
Dihedral |
| |
length(˚A) |
|
angle (o) |
|
angle (o) |
| |
|
|
|
|
|
| C1-C2 |
1.424 |
C1-C2-C4 |
106.85 |
C6-C1-C2-C4 |
-174.834 |
| C2-C4 |
1.419 |
C2-C1-C6 |
108.85 |
C2-C1-C6-C8 |
-8.963 |
| C1-C6 |
1.448 |
C1-C6-C8 |
107.56 |
C1-C2-C4-C8 |
6.05 |
| C6-C8 |
1.391 |
C10-C11-C14 |
93.45 |
C10-C11-C14-C16 |
-85.112 |
| C10-C11 |
1.471 |
C12-C10-C11 |
97.468 |
C12-C10-C11-C14 |
87.862 |
| C10-C12 |
1.427 |
C10-C12-C16 |
105.11 |
C10-C11-C14-C16 |
82.95 |
| C11-C14 |
1.366 |
Ni-C12-C10 |
72.433 |
C14-C16-C12-Ni |
-67.377 |
| C12-C16 |
1.427 |
|
|
|
|
| Ni-C2 |
1.976 |
|
|
|
|
Table 2: The selected bond length °A, bond angles and dihedral angles of the optimized Structure of nickelocene using DFT levels with B3LYP/6-31G (d) basis set.
| |
|
|
| Molecular properties |
cobaltocene |
nickelocene |
| RB-HF-LYP (eV) |
-1769.5679 |
-1650.74 |
| εHOMO(eV) |
-0.6452 |
-0.6427 |
| εLUMO(eV) |
-0.5626 |
-0.5614 |
| εLUMO-εHOMOenergy gap (eV) |
0.0826 |
0.0813 |
| Ionization potential(I in eV) |
0.6452 |
0.6427 |
| Electron affinity(A in eV) |
0.5626 |
0.5614 |
| Global hardness (ηineV) |
0.04129 |
0.04065 |
| Global softness (Sin eV) |
24.216 |
24.603 |
| Electro negativity (χineV) |
0.6039 |
0.6021 |
| Chemical potential (µin eV) |
-0.6039 |
-0.6021 |
| Dipole moment (µin Debye) |
1.695 |
1.931 |
| Mulliken charge distributions (M.C.D in e) |
± 1.13 |
± 1.692 |
Table 3: Important parametric properties of the molecules calculated at
the DFT/B3LYPLevels in 6-31G (d) basis set.
| MOs |
4s |
4px |
4py |
4pz |
3dx2-y2 |
3dz2 |
3dxy |
3dxz |
3dyz |
| |
χ41 |
χ42 |
χ43 |
χ44 |
χ45 |
χ46 |
χ47 |
χ48 |
χ49 |
| Φ20 |
- |
- |
- |
- |
- |
- |
- |
- |
0.2124 |
| Φ21 |
- |
- |
- |
- |
- |
- |
- |
0.2335 |
0.3224 |
| Φ22 |
- |
- |
- |
- |
- |
- |
0.2113 |
- |
- |
| Φ23 |
- |
- |
- |
- |
0.2507 |
0.4737 |
0.2664 |
0.2327 |
0.1436 |
| Φ24 |
- |
- |
- |
- |
0.5537 |
0.1378 |
0.4186 |
0.1448 |
0.2731 |
| Φ25 |
0.1198 |
- |
- |
- |
- |
0.5281 |
- |
0.3033 |
0.5717 |
| Φ26 |
- |
- |
- |
- |
0.2652 |
0.1081 |
0.5281 |
0.3717 |
0.3035 |
| Φ27 |
- |
- |
- |
- |
0.1445 |
- |
0.2931 |
0.2063 |
- |
| Φ28 |
- |
- |
- |
- |
0.1103 |
0.1402 |
0.2339 |
- |
0.2297 |
| Φ29 |
- |
- |
- |
- |
- |
0.2052 |
0.2116 |
0.1243 |
- |
| Φ30 |
- |
- |
- |
- |
- |
0.5131 |
0.2416 |
0.3996 |
0.1363 |
| Φ31 |
- |
- |
- |
- |
0.3741 |
- |
- |
0.3523 |
0.4225 |
| Φ36 |
0.3346 |
0.9288 |
- |
0.407 |
- |
- |
- |
- |
- |
| Φ37 |
0.3259 |
- |
0.8777 |
0.565 |
- |
- |
- |
- |
- |
| Φ38 |
0.6188 |
- |
- |
0.2691 |
- |
- |
- |
- |
- |
| Φ41 |
0.3195 |
- |
0.3626 |
0.4823 |
- |
- |
- - |
- |
- |
| Φ42 |
0.3148 |
- |
0.3549 |
0.3479 |
- |
- |
- |
- |
- |
| Φ46 |
0.2767 |
0.3026 |
- |
0.3002 |
- |
- |
- |
- |
- |
| Φ47 |
0.3329 |
- |
0.2698 |
0.2911 |
- |
- |
- |
- |
- |
| Φ50 |
0.7259 |
- |
0.2913 |
0.3794 |
- |
- |
- |
- |
- |
| Φ51 |
0.5741 |
0.5317 |
0.3413 |
0.7871 |
- |
- |
- |
- |
- |
| Φ54 |
- |
0.238 |
0.2945 |
0.2227 |
- |
- |
- |
- |
- |
| Φ55 |
0.3738 |
0.2889 |
0.3878 |
- |
- |
- |
- |
- |
- |
| Φ56 |
0.2297 |
- |
- |
- |
- |
- |
- |
2.3685 |
- |
N.B: orbitals having coefficient values above 0.10 have only been considered.
Table 4: Contributions of orbitals of cobalt and their summation values in the formation of molecular orbitals of cobaltocene.
Figure 1: Structure of cobaltocene and nickelocene molecules.
Figure 2: The optimized structures of cobaltocene and
nickelocene molecules.
Figure 3: The Milliken charge distributions of cobaltocene and
nickelocene Molecules.
Figure 4: The left side of HOMO and the right side of LUMO
surfaces of cobaltocene and nickelocene molecules
down of the column respectively.
Out of 59 molecular orbital Eigen values of cobaltocene we shall
discuss only 24 of them described in Table 4, for cobalt orbitals
and Table 6, for carbon orbitals. The first 12MOs are Φ20 −Φ31
formed by various 3d orbital of cobalt and 2pz orbital of (C5H5)-.
These orbital with energies in the range of -0.86738 to -0.56256
eV are the most stable molecular orbitals. The next 12 molecular
orbitals are Φ36-Φ38, Φ41-Φ42, Φ46-Φ47, Φ50-Φ51; Φ54-Φ55
and Φ56 have contributions from vacant 4s, 4px, 4py and 4pz
orbital of cobalt and 2pz orbital of carbon. These MOs with
energies between -0.37685 to -0.03634 eV are comparatively
less stable. To examine the extent of involvement of 3d, 4s,
and, 4p orbital in the formation of molecular orbital the values
of coefficient of each orbital have been added to see the total
involvement in all the 24 molecular orbital shown in Table 5.
| Atomic orbital’s of Co |
Sum of contributions of orbital’s of Co |
Sum of reactivity |
| 4s |
4.5465 |
0.2199 |
| 4p x |
2.29 |
0.4367 |
| 4p y |
3.1799 |
0.3145 |
| 4p z |
4.0518 |
0.2468 |
| 3dx2-y2 |
1.6985 |
0.58875 |
| 3d2z |
2.1062 |
0.4748 |
| 3dxy |
2.4046 |
0.4159 |
| 3dxz |
2.3685 |
0.4222 |
| 3dyz |
2.6152 |
0.3824 |
Table 5: Sum of contributions and reactivity of atomic orbital’s of cobalt
in the formation of molecular orbitals of cobaltocene.
| MOs |
1C |
2C |
4C |
6C |
8C |
10C |
11C |
12C |
14C |
16C |
| |
χ4 |
χ 8 |
χ 12 |
χ 16 |
χ 20 |
χ 24 |
χ 28 |
χ 32 |
χ 36 |
χ 40 |
| Φ20 |
0.3731 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ21 |
- |
- |
- |
- |
- |
0.3214 |
- |
- |
- |
- |
| Φ22 |
- |
- |
- |
- |
- |
- |
- |
0.3794 |
- |
- |
| Φ23 |
0.1117 |
- |
- |
0.2610 |
- |
0.1332 |
- |
0.1701 |
0.1118 |
- |
| Φ24 |
- |
- |
0.1153 |
- |
- |
0.1220 |
- |
0.1060 |
- |
- |
| Φ25 |
- |
- |
- |
0.1338 |
- |
0.1464 |
- |
- |
- |
- |
| Φ26 |
- |
0.1818 |
- |
0.1756 |
- |
- |
- |
- |
- |
0.1472 |
| Φ27 |
- |
0.3281 |
0.2463 |
0.2716 |
0.2181 |
0.2369 |
0.2113 |
- |
- |
0.1374 |
| Φ28 |
0.2825 |
- |
0.2548 |
0.1501 |
0.1591 |
0.2836 |
0.2755 |
- |
0.2362 |
- |
| Φ29 |
- |
0.2024 |
0.1605 |
- |
0.1336 |
0.1537 |
0.1515 |
- |
- |
0.3061 |
| Φ30 |
0.2899 |
- |
0.2750 |
0.1276 |
0.2474 |
0.2537 |
- |
0.2649 |
0.1558 |
0.2073 |
| Φ31 |
0.2835 |
0.3653 |
- |
0.2853 |
0.2495 |
- |
0.2476 |
- |
- |
- |
| Φ36 |
- |
0.238 |
- |
- |
- |
- |
- |
0.2304 |
- |
- |
| Φ37 |
- |
- |
- |
0.2413 |
- |
- |
0.2212 |
- |
- |
- |
| Φ38 |
- |
- |
- |
0.5191 |
- |
0.1146 |
- |
0.1874 |
0.2835 |
- |
| Φ41 |
0.2925 |
- |
- |
0.5471 |
0.2847 |
- |
- |
- |
- |
- |
| Φ42 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ46 |
- |
- |
- |
- |
- |
0.2580 |
- |
- |
0.260 |
- |
| Φ47 |
- |
- |
- |
0.3743 |
- |
- |
- |
- |
- |
- |
| Φ50 |
- |
- |
- |
0.2733 |
- |
- |
- |
- |
- |
0.2233 |
| Φ51 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0.5771 |
| Φ54 |
- |
- |
- |
- |
0.2218 |
- |
- |
- |
- |
- |
| Φ55 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ56 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| S |
1.6332 |
1.3124 |
1.0519 |
3.3601 |
1.5142 |
2.0235 |
1.1071 |
1.3382 |
1.0473 |
1.5984 |
Table 6: Contributions of 2pz orbitals of carbon atoms in (C5H5)- in the formation of molecular orbitals of cobaltocene.
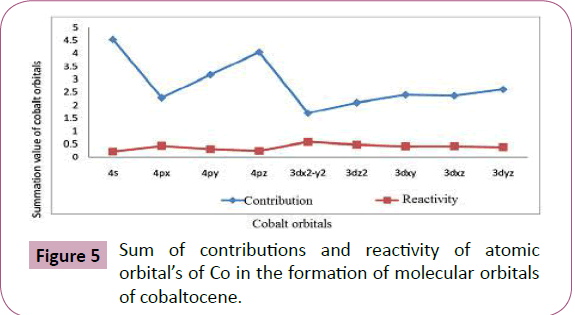
The summation values given in Table 5 and plotted in Figure 5 show the total contributions from each atomic orbital. It is clearly
indicated that 4s orbital has the maximum involvement out of 4s
and 4p orbital and 3dyz orbital has the maximum involvement out
of 3d orbitals. The sequence from the two series is given below:
Figure 5: Sum of contributions and reactivity of atomic
orbital’s of Co in the formation of molecular orbitals
of cobaltocene.
4s>4pz>4py>4px and
3dyz>3dxy>3dxz>3d2z>3dx2-y2 1
Sum of contributions of atomic orbitals of cobalt in the formation
of molecular orbitals of cobaltocene is shown in Table 5 and Figure 5. That the sum of contributions of 3dx2-y2 orbital in the
formation of molecular orbitals is least out of the 3d orbitals and
4px orbital is least out of 4s and 4p orbitals. Hence 3dx2-y2 and
4px are comparatively free for complex formations. The exact
order of availability of atomic orbitals of Co in cobaltocene for
complex formation is given below:
4px>4py >4pz >4s and
3dx2-y2>3dz2>3dxz>3dxy>3dyz 2
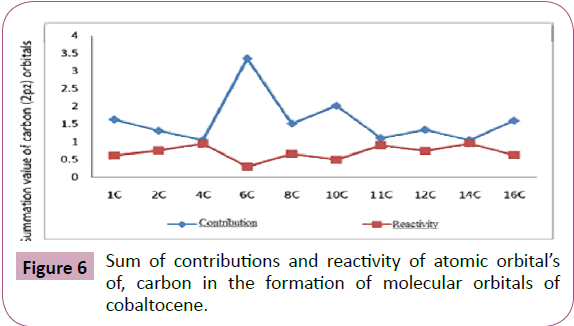
The summation values shown in Table 7 and Figure 6, clearly
indicates that contribution of 2pz orbital of 6C has the maximum
involvement out of the ten carbon atoms in (C5H5)- The sequence
from the series are given below:
| Atomic orbital’s of carbon |
Sum of contribution of carbon orbital’s |
Sum of reactivity |
| 1C |
1.6332 |
0.6123 |
| 2C |
1.3124 |
0.7619 |
| 4C |
1.0519 |
0.9507 |
| 6C |
3.3601 |
0.2976 |
| 8C |
1.5142 |
0.6604 |
| 10C |
2.0235 |
0.4942 |
| 11C |
1.1071 |
0.9033 |
| 12C |
1.3382 |
0.7473 |
| 14C |
1.0473 |
0.9549 |
Table 7: Sum of contributions and reactivity of atomic orbitals of carbon (2pz) in the formation of molecular orbitals in cobaltocene.
6C>10C>1C>16C>8C>12C>2C>11C>4C>14C 3
Sum of contributions of atomic orbitals of carbon (2pz) in the
formation of molecular orbitals of cobaltocene is shown in Table 7 and Figure 6 where the sum of contribution of 14C of
2pz orbital’s in the formation of molecular orbital’s are least out
of the ten carbon atoms. Hence 14C are comparatively free for complex formations. The exact order of availability of carbon
atom for complex formation is given below:
Figure 6: Sum of contributions and reactivity of atomic orbital’s
of, carbon in the formation of molecular orbitals of
cobaltocene.
14C>4C>11C>2C>12C>8C>16C>1C>10C>6C 4
Out of 59 molecular orbital Eigen values of nickelocene we shall
discuss only 25 of them described in Table 8, for nickel orbitals
and Table 10, for carbon orbitals. The first 14 MOs are Φ15-Φ16,
Φ18-Φ20, Φ21 and Φ23-Φ30, are formed by various 3d and 2pz
orbitals of (C5H5)−. These orbitals with energies in the range of
-9.9338 to - 0.64271eV are the most stable molecular orbital
between nickel and 2pz orbital of (C5H5)−. The next eleven MOs
i.e. Φ36-Φ40, Φ42-Φ43, Φ50, Φ53, Φ54 and Φ59 are formed
by interaction of 4s, 4px, 4py and 4pz orbital of metal and 2pz
orbital of carbon of (C5H5)−. These MOs with energies in the
range -0.56142 to -0.10622 eV are comparatively less stable. To
examine the extent of involvement of 3d, 4s, 4p and 2pz orbitals in the formation of molecular orbitals the values of coefficient of
each orbital are tabulated in Table 8.
| MOs |
4s |
4p x |
4py |
4pz |
3dx2-y2 |
3dz2 |
3dxy |
3dxz |
3dyz |
| |
χ41 |
χ42 |
χ43 |
χ44 |
χ45 |
χ46 |
χ47 |
χ48 |
χ49 |
| Φ15 |
- |
- |
- |
- |
- |
- |
- |
0.3209 |
0.3365 |
| Φ16 |
- |
- |
- |
- |
- |
- |
- |
0.2294 |
0.1991 |
| Φ18 |
- |
- |
- |
- |
- |
- |
0.3605 |
- |
- |
| Φ19 |
- |
- |
- |
- |
- |
- |
0.3125 |
- |
- |
| Φ20 |
- |
- |
- |
- |
0.5297 |
- |
- |
- |
- |
| Φ21 |
- |
- |
- |
- |
0.3029 |
- |
- |
- |
- |
| Φ23 |
- |
- |
- |
- |
0.3279 |
- |
- |
- |
0.1838 |
| Φ24 |
- |
- |
- |
- |
- |
- |
0.3849 |
0.4986 |
0.2011 |
| Φ25 |
- |
- |
- |
- |
0.5232 |
0.3369 |
- |
0.2396 |
0.2782 |
| Φ26 |
- |
- |
- |
- |
- |
0.7408 |
- |
- |
0.4949 |
| Φ27 |
- |
- |
- |
- |
- |
- |
0.6697 |
- |
0.2358 |
| Φ28 |
- |
- |
- |
- |
0.1702 |
- |
- |
- |
- |
| Φ29 |
- |
- |
- |
- |
- |
0.2012 |
- |
- |
- |
| Φ30 |
- |
- |
- |
- |
- |
0.2979 |
0.1819 |
0.4786 |
0.3521 |
| Φ36 |
- |
0.5037 |
0.5563 |
0.2729 |
- |
- |
- |
- |
- |
| Φ37 |
0.4088 |
0.3254 |
0.2960 |
- |
- |
- |
- |
- |
- |
| Φ38 |
0.6732 |
0.2423 |
0.3695 |
- |
- |
- |
- |
- |
- |
| Φ39 |
- |
0.7569 |
0.3868 |
0.3261 |
- |
- |
- |
- |
- |
| Φ40 |
- |
0.2201 |
0.3706 |
- |
- |
- |
- |
- |
- |
| Φ42 |
- |
0.3487 |
0.3318 |
- |
- |
- |
- |
- |
- |
| Φ43 |
- |
0.6971 |
0.7470 |
- |
- |
- |
- |
- |
- |
| Φ50 |
0.7225 |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ53 |
- |
0.3020 |
- |
0.2283 |
- |
- |
- |
- |
- |
| Φ54 |
0.6421 |
0.4372 |
0.5633 |
- |
- |
- |
- |
- |
- |
| Φ59 |
- |
0.4436 |
0.7620 |
1.1256 |
- |
- |
- |
- |
- |
| S |
2.4466 |
4.277 |
4.3833 |
1.9529 |
1.8539 |
1.5768 |
1.9095 |
1.7671 |
2.2815 |
N.B: Orbitals having coefficient values above 0.10 haveonly been considered.
Table 8: Contributions of orbitals of nickel in the formation of molecular orbitals of nickelocene.
| MOs |
1C |
2C |
4C |
6C |
8C |
10C |
11C |
12C |
14C |
16C |
| |
χ4 |
χ 8 |
χ 12 |
χ 16 |
χ 20 |
χ 24 |
χ 28 |
χ 32 |
χ 36 |
χ 40 |
| Φ15 |
- |
- |
- |
- |
- |
- |
- |
- |
0.1678 |
- |
| Φ16 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ18 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ19 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ20 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ21 |
- |
- |
- |
0.2019 |
- |
- |
- |
- |
- |
0.1986 |
| Φ23 |
- |
- |
- |
0.2182 |
- |
- |
0.1815 |
- |
- |
- |
| Φ24 |
- |
- |
- |
- |
0.1692 |
- |
- |
0.1544 |
- |
0.1707 |
| Φ25 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0.2018 |
| Φ26 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ27 |
- |
- |
0.2142 |
- |
- |
0.1666 |
- |
- |
- |
- |
| Φ28 |
0.2818 |
- |
0.3050 |
- |
0.2252 |
0.4088 |
0.2304 |
- |
0.3089 |
- |
| Φ29 |
- |
0.4189 |
0.2037 |
0.2561 |
0.2707 |
- |
0.3600 |
- |
0.2006 |
0.3209 |
| Φ30 |
0.3293 |
- |
0.3232 |
0.1812 |
0.1505 |
0.3096 |
- |
0.3219 |
0.1864 |
0.2300 |
| Φ36 |
- |
- |
0.2106 |
- |
- |
0.2172 |
- |
- |
0.3003 |
- |
| Φ37 |
0.2124 |
0.2023 |
- |
- |
- |
0.2123 |
- |
- |
- |
- |
| Φ38 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ39 |
- |
- |
- |
- |
- |
- |
0.2029 |
0.2264 |
- |
- |
| Φ40 |
- |
- |
- |
- |
0.4239 |
- |
- |
- |
- |
- |
| Φ42 |
- |
- |
- |
- |
0.2725 |
- |
- |
- |
- |
- |
| Φ43 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Φ50 |
- |
- |
- |
- |
0.4095 |
- |
- |
- |
- |
0.3640 |
| Φ53 |
- |
- |
- |
0.2129 |
- |
- |
- |
- |
- |
- |
| Φ54 |
- |
- |
- |
- |
- |
0.2723 |
- |
- |
- |
- |
| Φ59 |
- |
- |
- |
- |
0.2227 |
- |
- |
- |
- |
- |
| S |
0.8235 |
0.6212 |
1.2567 |
1.0703 |
2.1442 |
1.5868 |
0.9748 |
0.7027 |
1.164 |
1.486 |
N.B: orbital having coefficient value above, 0.10 have onlybeen considered.
Table 10: Contributions of 2pz orbitals of carbon atoms in (C5H5)- in the formation of molecular orbitals of nickelocene.
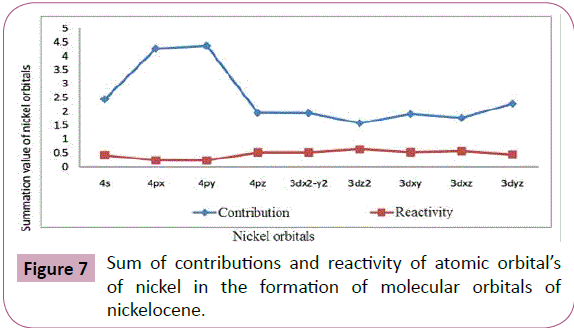
The summation values given in Table 9 and plotted in Figure 7 show the total contributions from each atomic orbital. It is clearly
indicated that 4py orbital has the maximum involvement out of
4s and 4p orbital and 3dyz orbital has the maximum involvement
out of 3d orbitals. The sequence from the two series is given
below:
Figure 7: Sum of contributions and reactivity of atomic orbital’s
of nickel in the formation of molecular orbitals of
nickelocene.
| Atomic orbital’s of Ni |
Sum of contributions of orbital’s of Ni |
Sum of reactivity |
| 4s |
2.4466 |
0.4087 |
| 4px |
4.277 |
0.2338 |
| 4py |
4.3833 |
0.2281 |
| 4pz |
1.9529 |
0.5121 |
| 3dx2-y2 |
1.9439 |
0.5144 |
| 3d2z |
1.5768 |
0.6342 |
| 3dxy |
1.9095 |
0.5237 |
| 3dxz |
1.7671 |
0.5657 |
| 3dyz |
2.2815 |
0.4383 |
Table 9: Sum of contributions and reactivity of atomic orbital’s of nickel in the formation of molecular orbitals of nickelocene.
4py>4px>4s>4pz and
3dyz>3dx2-y2>3dxy>3dxz>3d2z 5
Sum of contributions of atomic orbitals of nickel in the formation
of molecular orbitals of nickelocene is shown in Table 9 and Figure7 that the sum of contributions of 3d2z orbital in the
formation of molecular orbitals is least out of the 3d orbitals and
4pz orbital is least out of 4s and 4p orbitals. Hence 3d2z and 4pz
are comparatively free for complex formations. The exact order
of availability of atomic orbitals of Ni in nickelocene for complex
formation is given below:
4pz>4s>4px>4pyand
3dyz>3dxz>3dxy>3dx2-y2>3dyz 6
The summation values shown in Table 11 and Figure 8 clearly
indicates that contribution of 2pz orbital of 8C has the maximum
involvement out of the ten carbon atoms in (C5H5)−. The sequence
from the series are given below:
Figure 8: Sum of contributions and reactivity of atomic orbital’s of,
Ni in the formation of molecular orbitals of nickelocene.
8C >10C >16C >4C >14C >6C >11C >1C >12C >2C 7
Sum of contributions of atomic orbitals of carbon (2pz) in the
formation of molecular orbitals of nickelocene is shown in Table
11 and Figure 8 where the sum of contribution of 2C of 2pz
orbital’s in the formation of molecular orbital’s are least out of the
ten carbon atoms. Hence 2C are comparatively free for complex
formations. The exact order of availability of carbon atom for
complex formation is given below:
| Atomic orbital’s of carbon |
Sum of contribution of carbon orbital’s |
Sum of reactivity |
| 1C |
|
|
0.8235 |
|
|
|
1.2143 |
|
| 2C |
|
|
0.6212 |
|
|
|
1.6098 |
|
| 4C |
|
|
1.2567 |
|
|
|
0.7957 |
|
| 6C |
|
|
1.0703 |
|
|
|
0.9344 |
|
| 8C |
|
|
2.1442 |
|
|
|
0.4667 |
|
| 10C |
|
|
1.5868 |
|
|
|
0.6302 |
|
| 11C |
|
|
0.9748 |
|
|
|
1.0259 |
|
| 12C |
|
|
0.7027 |
|
|
|
1.4231 |
|
| 14C |
|
|
1.164 |
|
|
|
0.8591 |
|
| 16C |
|
|
1.486 |
|
|
|
0.6729 |
|
Table 11: Sum of contributions and reactivity of atomic orbitals of carbon (2pz) in the formation of molecular orbitals in nikelocene.
2C>12C>1C>11C>6C>14C>4C>16C>10C>8C 8
The total involvement in relation to the bonding between
metal orbital derived from coefficient values are 24.2528 in
cobaltocene, and 22.8486 in nickelocene hence cobaltocene is
more stable than nickelocene. The total involvement in relation
to the bonding between 2pz orbital of the ten carbon atoms of
both ligands of (C5H5)- 15.986 and 15.529 in cobaltocene and
nickelocene respectively, hence cobaltocene is more stable than
nickelocene. The total involvement of 3d, 4s and 4p orbitals of
metal and 2pz orbitals of the ten carbon atoms of both ligands of
(C5H5)− in cobaltocene and nickelocene respectively are 40.2388,
and 38.3776 hence we can conclude that cobaltocene is more
stable than nickelocene.
Population Analysis
The contribution of electrons in each occupied MO is calculated
by using the population analysis method introduced by Mullikan
[24-26]. This method apportions the electrons of n-electron
molecule in to net population nr in the basis function χ(r). Let
there be ni electrons in the MO Φi (ni=0, 1, 2) and letnri symbolize
the contribution of electrons in the MO Φi to the net population
in χr we have:
nri=nic2ri 9
Where, cri is the coefficient of atomic orbital for the ith MO
r=1-30 in cobaltocene and nickelocene. Eq (9) has been solved for, 60 electrons of 30 molecular orbitals in cobaltocene and
nickelocene. Each MOs has two electrons in cobaltocene and
nickelocene but (the 30th MOs of cobaltocene and nickelocene
have only one electron). The coefficient of atomic orbital cri is
treated as Eigen vector value [24-26]. Value less than 0.1 have
negligible contributions and are omitted in the calculations. Only
3dorbitals of metal and 2pz orbitals of carbon are considered in
the calculation. The summation value of population analysis of
these orbitals is shown in Table 12 of cobaltocene, and Table 13 of nickelocene. It is indicated that in MOs 1-19 of cobaltocene,
in MOs 1-14 of nickelocene only 2s, 2py and 2px electrons of
carbon have contributions in the formation of molecular orbital
of cobaltocene and nickelocene hence are out of discussion.
The summation value of population analysis of these orbitals
to contribute electrons in the formation of molecular orbital is
shown Tables 12 and 13 the result of the population analysis
shows that only 2pz orbitals of carbon of (C5H5)− and 3d orbitals of
metal provide electrons to MOs of cobaltocene, and nickelocene.
| MOs |
No. of atomic orbitals |
Eigenvector (cri) |
No. of electrons (ni) |
Net population (nri) |
| Φ20 |
2 |
0.5855 |
4 |
0.3344 |
| Φ21 |
3 |
0.8773 |
6 |
0.5154 |
| Φ22 |
2 |
0.5907 |
4 |
0.3470 |
| Φ23 |
10 |
2.1549 |
20 |
1.2661 |
| Φ24 |
8 |
1.8713 |
16 |
1.0994 |
| Φ25 |
6 |
1.8031 |
12 |
1.0593 |
| Φ26 |
8 |
2.6812 |
16 |
1.3475 |
| Φ27 |
10 |
2.2936 |
20 |
1.1527 |
| Φ28 |
11 |
2.3559 |
22 |
1.1829 |
| Φ29 |
9 |
1.6489 |
18 |
0.8279 |
| Φ30 |
12 |
2.9759 |
12 |
0.9391 |
Summation value of population analysis, (nri) of occupied molecular orbital of cobaltocene is 10.0715.
Table 12: The sum of contribution of electrons 3d orbitals of cobalt and2pzorbitals ofcarbon in the formation ofmolecular orbitals ofcobaltocene.
| MOs |
No. of atomic orbitals |
Eigenvector (cri) |
No. of electrons (ni) |
Net population (nri) |
| |
|
|
|
|
| Φ15 |
3 |
0.8252 |
6 |
0.4884 |
| Φ16 |
2 |
0.4285 |
4 |
0.2537 |
| Φ18 |
1 |
0.3605 |
2 |
0.2134 |
| Φ19 |
1 |
0.4718 |
2 |
0.2793 |
| Φ20 |
1 |
0.5297 |
2 |
0.4163 |
| Φ21 |
3 |
0.7034 |
6 |
0.5529 |
| Φ23 |
4 |
1.1606 |
8 |
0.9122 |
| Φ24 |
6 |
1.5789 |
12 |
1.2410 |
| Φ25 |
5 |
1.7438 |
10 |
1.0979 |
| Φ26 |
2 |
1.2357 |
4 |
0.7780 |
| Φ27 |
4 |
0.9055 |
8 |
0.5701 |
| Φ28 |
7 |
1.9303 |
14 |
1.1425 |
| Φ29 |
8 |
2.2311 |
16 |
1.3206 |
| Φ30 |
12 |
1.3426 |
24 |
0.7947 |
Summation value of population analysis, (nri) of occupied molecular orbital of nickelocene is, 10.0609.
Table 13: The Sum of contribution of electrons, 3d orbitals of nickel and, 2pz orbitals of carbon in the formation ofmolecular orbitals of nickelocene.
Conclusion
We studied the electronic structure and geometry optimization
of cobaltocene and nickelocene molecules using DFT/B3LYP with
the basis set of 6-31G (d) calculations. We found that orbitals
corresponding to the Eigen values (energy ranges -0.86738
to -0.56256 eV in cobaltocene and -9.90743 to -0.64271 eV in nickelocene) formed between 3d orbitals and 2pz orbitals are the
most stable molecular orbitals. The less stable orbitals are in the
energy ranges of -0.37685 to -0.03634 eV in cobaltocene and in
-0.56142 to -0.10622 eV nickelocene. Eigenvectors of cobaltocene
and nickelocene show that the first 12 MOs in cobaltocene 14 MOs
nickelocene are formed by various 3d orbitals of metal and 2pz
orbital of carbon of (C5H5)− and the most stable MOs. The next 12
MOs in cobaltocene and 11 MOs of nickelocene are formed by the
interaction of 4s and 4p orbitals of metal and2pz orbital of carbon
of (C5H5)− and these MOs are comparatively less stable orbitals.
Out of the 3d orbitals of cobaltocene and nickelocene molecules
the 3dyz orbitals have maximum involvement in the formation
of molecular orbitals, whereas the 4s orbital out of 4s and 4p
orbital of cobalt and 4py orbital out of 4s and 4p orbital of nickel
show maximum involvement, in the order of4s>4pz>4py>4px and
3dyz>3dxy>3xz>3dz2>3dx2-y2 in cobaltocene, and 4py>4px>4s>4pz
and 3dyz>3dx2-y2>3dxy>3dxz>3dz2 in nickelocene.. The total
involvement in relation to the bonding between metal orbital
derived from coefficient values are 24.2528 in cobaltocene and
22.8486 in nickelocene hence cobaltocene is more stable than nickelocene. The total involvement in relation to the bonding
between 2pz orbital of the ten carbon atoms of both ligands
of (C5H5) 15.986, and 15.529 in cobaltocene and nickelocene
respectively, hence cobaltocene is more stable than nickelocene.
As a summary, the total involvement of 3d, 4s and 4p orbitals of
metal and 2pz orbitals of the ten carbon atoms of both ligands of
(C5H5)− in cobaltocene and nickelocene respectively are 40.2388
and 38.3776 hence we can conclude that cobaltocene is more
stable than nickelocene. This is in support of the results shown in
terms of the parameters like dipole moment, HOMO-LUMO gap, Ionization potential etc. discussed in the above. The population
analysis shows that only 2pz orbitals of carbon of (C5H5) -and 3d
orbitals of metal provide electrons to MOs of cobaltocene and
nickelocene.
Acknowledgement
The author acknowledges Dr. Hagos Woldeghebriel for his
advising, ideas, guidance, enjoyable discussion and unforgettable
support throughout my study.
References
- Cotton FA, Wilkinson G, Gaus PL (2001) Basic Inorganic Chemistry, (3rd edn), Wiley and Sons, Asia.
- Girolami S,Rauchfuss TB, Angelici RJ (1999) Synthesis and Technique in Inorganic Chemistry, CA: University Science Books- Mill Valley.
- Davidson ER (2000) Handbook of Metathesis. Chem Rev 100: 351-352.
- Davidson ER (1991) Molecular Modeling: Basic Principles and Applications. Chem Rev.
- Nalewajski RF (1996) Topics in current chemistry. Heidelberg: Berlin: Springer-Verlag.
- Parr RG, Yang W (1989) Density Functional Theory of atoms and molecules. Oxford University Press, New York.
- Labanowski J, Anzelm J (1991) Density Functional Methods in Chemistry. Springer-Verlag, Heidelberg.
- Ziegler T (1991) Approximate density functional theory as a practical tool in molecular energetics and dynamics. Chem Rev 91: 651-667
- Szasz L (1986) Pseudopotential Theory of Atoms and Molecules. Wiley & Sons, New York.
- Krauss M, Stevens WJ (1984) Effective Potentials in Molecular Quantum Chemistry. Ann Rev PhysChem 35: 357.
- Durand P, Malrieu JP (1987)New Theoretical Concepts for Understanding Organic Reactions.AdvChemPhys67: 321.
- Cundari TR, Benson MT, Lutj ML, Sommerer SO (1996) Reviews in Computational Chemistry.LipkowitzKB, Boyd DB (Eds.), VCH: New York.
- Mehrotra RC, Singh A (1992) Organometallic Chemistry, Wiley Eastern Ltd.
- Levine IN (2000) Quantum Chemistry, (5th edn.) Prentice Hall: New Jersey.
- Cleary DA, Francis AH (1985) Electron spin resonance spectra of cobaltocene intercalated cadmium phosphorus sulfide (CdPS3) layered host lattices. J PhysChem 89: 97-100.
- Byszewski PK,Antonova E,Kowalska J,Radomska,Baran J (2003)Guide for authors - Chemical Physics Letters. ChemPhysLett.
- Elschenbroich C, Salzer A (1991) Organometallics: A Concise Introduction, VCH: Weinheim, Germany.
- Wilkinson G, Pauson PL, Cotton FA (1954)Chiral Ferrocenes in Asymmetric Catalysis: Synthesis and Applications. J Am ChemSoc76:1970-1974.
- Schachtschneider JH,Prins J,Ros P (1967)The chemistry of nickelocene.InorgChim Act 1: 462.
- David ER (2000) Cross-Reactive Chemical Sensor Arrays - Chemical Reviews. Chem Rev 100: 2595-2626
- Khan G (2011) Molecular mechanics and Quantum Chemistry Study ofCobaltocene andNickelocene. Archives of Physics Research 3: 297-310.
- Sahu VK, Khan G, Verma RN, Singh PP (2010) Complexes of Cobaltocene: AnEffective Atomic Softness and Fukui Function Based Study. Journal of Pharmaceutical, Biological and Chemical Sciences 1: 535-544.
- Khan G (2011) Molecular Mechanics Based Study on Molecular and Atomic Orbital of Nickelocene. J ApplChem Res 10: 66-84.
- Kahn G, Rajendra PT (2011) Study of MolecularOrbitals of Ruthenium (II) Bromide Based on Molecular Mechanics.Applied Science Research 3: 483-492.
- Kahn G (2011) Comparative Study of Molecular Orbitals of Cobaltocene and Nickelocene Based on Molecular Mechanics.Applied Science Research 3: 297-310.
- Singh PP, Mishra P and Singh JP (2006) Molecular mechanics and quantum chemistry based study of cobalt-thiazolidinedione complexes.34: 215-224.