Research Article - (2025) Volume 9, Issue 1
Characterization and Evaluation of the Antibacterial Activity of Zinc Oxide Nanoparticles (ZnO NPs) Synthesized from the Latex of Synadenium grantii
Daniel K. Tirop1,
Lemeitaron Njenga1*,
Ayabei Kiplagat1,
Grace Lagat1 and
Martin O. Onani2
1Department of Chemistry and Biochemistry, University of Eldoret, Eldama Ravine, Kenya
2Department of Chemistry, University of the Western Cape, Bellville, South Africa
*Correspondence:
Lemeitaron Njenga, Department of Chemistry and Biochemistry, University of Eldoret, Eldama Ravine,
Kenya,
Email:
Received: 15-May-2024, Manuscript No. IPNNR-24-19893;
Editor assigned: 17-May-2024, Pre QC No. IPNNR-24-19893 (PQ);
Reviewed: 01-Jun-2024, QC No. IPNNR-24-19893;
Revised: 05-Mar-2025, Manuscript No. IPNNR-24-19893 (R);
Published:
12-Mar-2025, DOI: 10.12769/IPNNR.25.9.36
Abstract
The continuously increasing incidences of bacterial and infectious diseases are open threats to the sustainable survival of animals and humans. For the last two decades, the demands for eco-friendly nanomaterials as modern therapeutic agents have increased. This is the reason why, as opposed to using hazardous chemicals, researchers in recent times have concentrated on simple, green, sustainable and affordable ways to create nanoparticles. This study aimed to biosynthesize Zinc Oxide Nanoparticles (ZnO NPs) using the latex of Synadenium grantii through a rapid and eco-friendly approach. The formation of ZnO NPs was confirmed by UV-Visible (UV-VIS) spectroscopy, X-Ray Diffraction (XRD) and Fourier Transforms Infrared (FTIR) spectroscopy. The XRD pattern shown that the NPs were wurtzite in phase with an average diameter of 27 nm. The synthesized product demonstrated great potential as an anti-bacterial agent as tested on Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). The study has indicated that ZnO NPs, stabilized by metabolites in latex had a better anti-bacterial effect compared to the latex extract.
Keywords
Green nanoparticles; Synthesis; Latex; Synadenium grantii; ZnO; Anti-bacteria
Introduction
The biomedical field is very interested in the synthesis of zinc oxide nanoparticles with antibacterial properties, especially those that use green biological entities. Metal oxide nanoparticles have been synthesized using a variety of physical and chemical processes. Nevertheless, chemical synthesis carries a number of potential risks, such as cytotoxicity, genotoxicity and carcinogenicity. Furthermore, the end products of these processes cause harmful, poisonous compounds to adhere to the surface of the generated NPs, reducing their usefulness for medical purposes [1]. Green biosynthesis methods offer a safer approach and are gaining momentum. The procedures for synthesis of nanoparticles are environmentally friendly, particles synthesized possess exceptional, chemical, optical [2], biomedical [3] and electronic properties [4] which can be easily tuned just like those from chemical means; these biological procedures involve the use of organisms ranging from plants, bacteria and even fungi [5]. The phytochemicals in plants [6] and those both unicellular and multicellular organisms have the ability to synthesize and/or regulate the size of NPs [7]. The key advantages of this strategy for the environment are its low toxicity levels. Relationship building for pharmaceutical and other biomedical applications is one of the other advantages. Conventional methods for creating nanoparticles entail applying chemical and physical processes [8]. Zinc oxide is one of the several bio-genically synthesized nanoparticles. It belongs to a signi icant family of commercially feasible products that have special antibacterial, anticancer and magnetic qualities [9,10]. It also has the capacity to be both photo-catalytic and photo-oxidizing [11]. The Food and Drug Administration (FDA 21CFR182.8991) in the USA has classi ied zinc oxide as a "GRAS" (generally regarded as safe) substance, which is another appealing quality of the material. It is well known that a signi icant percentage of gram positive and gram negative bacteria are inhibited in their growth by ZnO nanoparticles [13]. Inorganic oxides nanoparticles are better in contrast to antimicrobial agents based on organic chemicals due to their extended shelf life, general stability and method of eradicating microorganisms [7]. Herbal remedies or the traditional use of plant extracts as medicine for a variety of ailments, have been around for a while. In Asian nations, natural compounds like Synadenium gran ii are commonly utilized for combating bacterial illnesses. But their efforts haven't yielded many results [14]. The current study investigated the biological synthesis of zinc oxide nanoparticles by incubating zinc acetate solution with Synadenium gran ii latex. The study assessed the antibacterial studies of the zinc oxide nanoparticles in comparison to the latex using the Kirby-Bauer disk diffusion susceptibility test against clinical isolates of gram positive (Staphylococcus aureus) and gram negative (Escherichia coli) bacterial strains in terms of zones of inhibition.
Materials and Methods
Chemicals and Reagents
Highly purity analytical grade chemicals of Zinc Acetate Dihydrate (Zn(CH3CO)2.2H2O), Sodium Hydroxide (NaOH) and distilled water were purchased at reno chemicals and lab equipment suppliers, Eldoret and used directly without further purifications.
Plant Extracts Collection and Preparation
Latex extracts from Synadenium grantii to be used in these studies was collected from Ziwa in Eldoret, Uasin-Gishu County, Kenya. The latex was collected by slitting the trunk of Synadenium grantii longitudinally; pure latex was collected in dark bottles and protected from light. The extract was refrigerated at 4°C until for further use. Diluted latex was prepared by mixing 1 mL of latex and 9 mL of DW (Distilled Water) then iltered ready for use in the synthesis of nanoparticles.
Biosynthesis of Zinc Oxide Nanoparticles
The synthesis method for Zinc Oxide Nanoparticles (ZnO NPs) was borrowed from [15] with few modification done. Briefly, 50 mL of distilled water and 1 mM of zinc acetate dehydrate were mixed. The mixture was stirred for 20 minutes using a magnetic stirrer. The resultant zinc acetate dehydrate solution was then mixed with 25 mL of plant extract and 1.8 M NaOH solution added drop wise. For one hour, the resulting reaction mixture was swirled. The solution's hue changed to yellow, indicating that ZnO NPs had formed. A centrifuge was used to separate the precipitate from the reaction liquid for 15 minutes at 8000 rpm at room temperature. The collected pellet for additional research was dried for 4 hours at 450°C and stored in airtight vials.
Characterization of Zinc Oxide Nanoparticles
Optical analysis: ZnO nanoparticles were subjected to analysis by a UV-visible spectrophotometer (UV-2450, Shimadzu) to determine their optical characteristics. A synthesized sample was suspended in sterile distilled water and scanned between wavelength’s 300 nm-500 nm.
The X-Ray Diffractoion (XRD) analysis: Pattern of biosynthesized ZnO NPs measurements were done on Bruker D8 Advance X-ray diffractometer system. The intensity of the diffracted Cu-Kα radiation (λ=0.154 nm, 40 kV and 40 mA) was measured in a 2θ range between 10o and 90o with an increment of 0.0194o.
Fourier Transform Infrared (FT-IR) spectroscopy analysis: To determine which bioactive phytoconstituents were present and responsible for the stabilization, decrease or control of the size of the ZnO nanoparticles, an FTIR spectrophotometer (Perkin-Elmer Spectrum 1000) was utilized. At ambient temperature, KBr pellets were used to acquire ZnO nanoparticle FTIR spectra in the wavelength range of 4000 cm-1-400 cm–1.
Anti-microbial activity: The procedure was adopted from [16] with several modifications. The ZnO NPs were examined for their antibacterial activity by growing both gram negative (E. coli) and gram positive (S. aureus) bacteria. The agar well diffusion technique methodology was utilized to ascertain the antibacterial activity of ZnO NPs. In a nutshell, the test organisms were inoculated for eight hours in Luria Bertani broth (pH 7.4). Cotton swabs that had been sterilized were used to seed the isolates onto Luria Bertani agar plates. To create wells, a sterile gel borer was used to bore holes in the agar surface (7 mm diameter). Separate wells were illed with varying amounts of ZnO NPs (25-100 mg/mL), latex extracts of Synedenium grantii and ampicillin (positive control). The plates were incubated at 37°C for 24 hrs.
Results and Discussion
Biosynthesis of Zinc Oxide Nanoparticles
The change in the reaction mixture's hue was very prominent and was observed within few minutes. This was considered an initial signature of formation of ZnO NPs. In our study, a change of color from white to red then to light yellow indicated the synthesis of zinc oxide nanoparticles by latex extracts of Synedenium grantii [17].
Optical Properties
UV-Vis spectroscopy is a common and widely used technique to characterize the optical properties of synthesized NPs. The Figure 1 (a) represents the UV-visible absorption spectra of the green synthesized ZnO NPs. Zinc oxide nanoparticles' highest absorption peak was measured at 365 nm (with band gap of 3.213eV). The appearance of a single peak at approximately 365 nm signified the formulation of zinc oxide nanoparticles. This finding is in tandem with previous findings of biosynthesized zinc oxide nanoparticles that were found to be absorbed in 324 nm–390 nm range [18-20]. This highly red-shifted absorption maximum band (from the bulk usually around 373 nm) is an indication of nano sized ZnO NPs formation.
The optical band gap energy (Eg) of the nanoparticle was determined by fitting the absorption data using Tauc’s relation [5]:
αhυ=E(hυ−Eg)1/2 ………………………Eqn 1
Where hν is the photon energy, Eg is the direct band gap and E is a constant, α is the optical absorption coefficient and is obtained from the absorption data. As presented in Figure 1 (b), plotting (αhν)2 as a function of photon energy (hυ) and extrapolating the linear portion of the curve to zero absorption gives the value of the direct band gap (Eg). It was observed that the band-gap energy was 3.213eV which is closer to that of typical ZnO (3.33 eV).
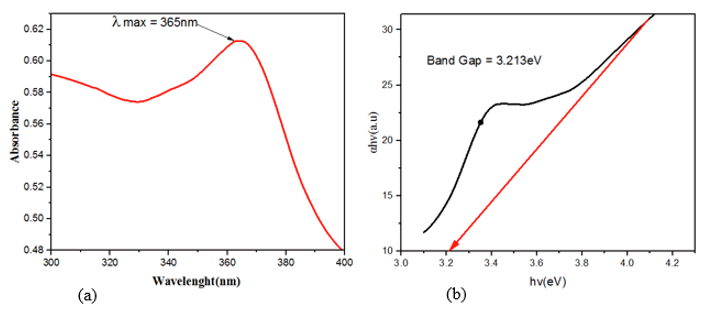
Figure 1: (a) UV-vis absorption spectra of ZnO nanoparticles biosynthesized by latex of Synadenium grantii and (b) Tauc plot representing the band gap energy of the biosynthesized ZnO nanoparticles.
XRD Analysis
The main peaks of the measured XRD patterns of the green synthesized zinc oxide nanoparticles (Figure 2) appear at 2θ values of 31.7, 34.5, 36.3, 47.5, 56.5, 62.8, 66.2, 67.9, 69.0, 72.6 and 76.9 o indexed to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1), (0 0 4) and (2 0 2) planes that confirmed wurtzite phases of the obtained nanoparticles (ZnO NPs) in correspondence to JCPDS card no: 00-065-3411. The sharp and narrow peaks indicate the good crystalline nature of ZnO NPs. The size of ZnO nanoparticles was estimated using the Scherrer’s equation. The zinc oxide nanoparticles that were biosynthesized had an average particle size of 27 nm. The X-ray diffraction spectra of ZnO NPs shown sharp diffraction peaks indicating the structural crystallinity.
FTIR Studies
The secondary metabolites are reported to be specified and involved in the bio-reduction of metal oxide NPs. Phenols, alkaloids, saponins, terpenes, triterpenes, ketones, sugars, fatty acids, proteins and aromatic acids are secondary metabolites in latex of Synadenium grantii and are proposed to serve as a bio-reductants in aqueous medium and exhibited greater biological activities including antioxidant and anticancer activity.
It can be reported that the Zn(CH3CO)2.2H2O (Zinc Acetate Dihydrate) is reduced to ZnO nanoparticles by phenols because of their massive -OH groups shown in the FT-IR spectra (Figure 2).
The FTIR spectra shows bands in 3446 cm-1 due to vibration stretch of the hydroxyl group of phenol. The C=O, C=O=C and C=C groups of heterocyclic compounds may act as a stabilizers (The –OH groups from the phenol in addition perform as a capping agent). Low intensity peak around 1541 cm-1 corresponded to C=C stretches of aromatized rings of polyphenols. Absorption at around 1541 cm-1 may also exhibit the linkage with ZnO NPs which may be assigned to the carbonyl stretch in proteins. Prominent lower peaks at 574 cm-1 are assigned to characteristic Zn-O stretch of ZnO NPs. Stabilization and encapsulation of bioformulated ZnO NPs results from –OH and C=O functional groups. It's also possible to draw the conclusion that the reduction process is brought about by the presence of protein and phenolic group molecules. So, the functional groups responsible for the reducing, capping or stabilizing agents were observed in vibrational bands in FTIR spectrum (Figure 2).
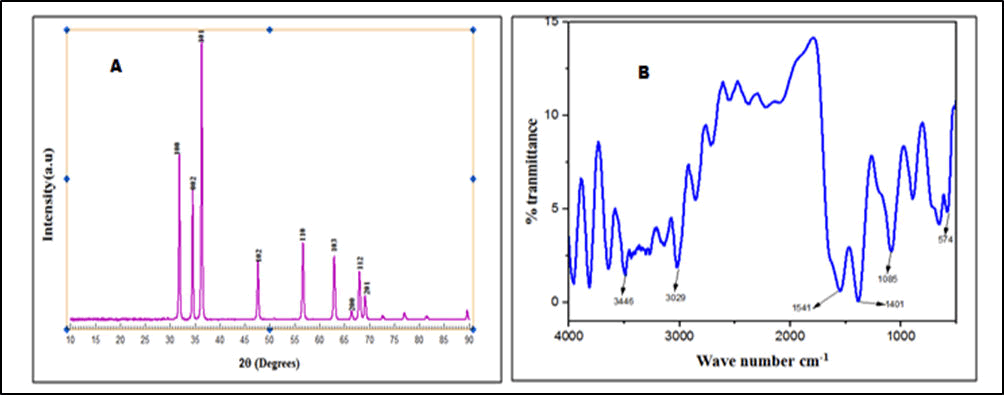
Figure 2: (a) X-ray diffraction patterns of zinc oxide nanoparticles and (b) FTIR profiles of zinc oxide nanoparticles.
The Antibacterial Activity
The anti-bacterial activities of the bioformulated ZnO nanoparticles were tested against two clinical isolates: Gram positive (Staphylococcus aureus) and gram negative (Escherichia coli) bacteria. The standard antibiotic ampicillin (positive control) was used to compare the activities. Figure 3 represents the zones of inhibition of the biosynthesized zinc oxide nanoparticles and ampicillin against the select microbes.
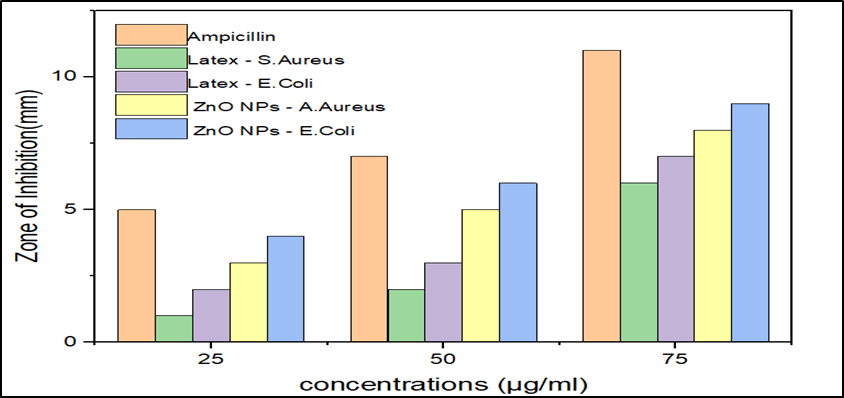
Figure 3: Bar graphs showing the inhibition zones by ZnO NPs against bacterial strains compared to latex of Synadenium grantii.
Conclusion
The study demonstrated successful formulation of ZnO nanoparticles using latex of Synadenium grantii. The ZnO nanoparticles were spherical in shape and in average nano sizes of 27 nm as demonstrated by XRD spectra. This approach has the benefit of being fast, eco-friendly, convenient for biosynthesis of ZnO nanoparticles. The band gap 3.213 eV was sufficient evidence for the fabrication of ZnO semiconductors. The investigation confirmed the stronger antibacterial potentials of the ZnO nanoparticles. This is because ZnO nanoparticles include antibacterial ROS (reactive oxygen species) in addition to being capped by anti-bacterial substances such phenols, alkaloids, saponins, terpenes, triterpenes, ketones, sugars, fatty acids, proteins and aromatic acids from latex extracts of Synadenium grantii. Thus, metal oxide-formulated nanoparticles containing latex extracts of Synadenium grantii are suitable for curing microbiological diseases.
Funding
University of Eldoret Annual research grants-UoE/D/DVPRE/ DRIV/NACO/074.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
We are grateful to the Chemistry Departments of the University of Eldoret for their cooperation with the laboratory work for this study. Prof. Martin Onani is especially appreciated for his XRD analysis.
Conflicts of Interest
The authors declare no conflict of interest
References
- Akhtar MS, Panwar J, Yun YS (2013) Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain Chem Eng. 1(6): 591–602.
[Google Scholar]
- Bala N, Saha S, Chakraborty M, Maiti M, Das S, et al. (2015) Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 5(7): 4993–5003.
[Google Scholar]
- Somu P, Paul S (2019) A biomolecule-assisted one-pot synthesis of zinc oxide nanoparticles and its bioconjugate with curcumin for potential multifaceted therapeutic applications. New J Chem. 43(30):11934–11948.
- Sorbiun M, Shayegan Mehr E, Ramazani A, Mashhadi Malekzadeh A (2018) Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochem Res. 3(1):1–16.
[Google Scholar]
- Muhammad W, Ullah N, Haroon M, Abbasi BH (2019) Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using: Papaver somniferum L. RSC Adv. 9(51):29541–29548.
[Crossref] [Google Scholar] [PubMed]
- Hameed S, Iqbal J, Ali M, Khalil AT, Abbasi BA, et al. (2019) Green synthesis of zinc nanoparticles through plant extracts: Establishing a novel era in cancer theranostics. Mater Res Express. 6(10).
[Google Scholar]
- Rauf MA, Owais M, Rajpoot R, Ahmad F, Khan N, et al. (2017) Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible: S. aureus skin infection in experimental animals. RSC Adv. 7(58):36361–36373.
[Google Scholar]
- Kanwar R, Rathee J, Salunke DB, Mehta SK (2019) Green nanotechnology-driven drug delivery assemblies. ACS Omega. 4(5):8804–8815.
[Crossref] [Google Scholar] [PubMed]
- Selim YA, Azb MA, Ragab I, El-azim MHMA (2020) Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci Rep. 1–9.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Lee JS, Kim D, Zhu L, Zhu L (2017) Exploration of zinc oxide nanoparticles as a multi-target and multi-functional anticancer nanomedicine. ACS Appl Mater Interfaces. 9(5):8804–8815.
[Crossref] [Google Scholar] [PubMed]
- Sivakumar P, Lee M, Kim YS, Shim MS (2018) Photo-triggered antibacterial and anticancer activities of zinc oxide nanoparticles. J Mater Chem B. 6(30).
[Crossref] [Google Scholar] [PubMed]
- Muthuvel A, Jothibas M, Manoharan C (2020) Effect of chemically synthesized compared to biosynthesized ZnO-NPs using Solanum nigrum leaf extract and their photocatalytic, antibacterial and in-vitro antioxidant activity. J Environ Chem Eng. 8(2):103705.
[Google Scholar]
- Abinaya C, Devi RM, Suresh P, Balasubramanian N, Muthaiya N, et al. (2020) Antibacterial and anticancer activity of hydrothermally-synthesized zinc oxide nanomaterials using natural extracts of neem, pepper and turmeric as solvent media. Nano Express.
[Google Scholar]
- Wang HB, Wang XY, Liu LP, Qin GW, Kang TG (2015) Tigliane diterpenoids from the Euphorbiaceae and Thymelaeaceae families. Chem Rev. 115(9):2975–3011.
[Crossref] [Google Scholar] [PubMed]
- Gilavand F, Branch K, Mirzaei SZ, Karkhane M, Marzban A (2021) Green synthesis of zinc nanoparticles using aqueous extract of Magnoliae officinalis and assessment of its bioactivity potentials. Biochem Res Int.
[Google Scholar]
- Radhakrishnan R, Liakath F, Khan A, Muthu A (2021) Green synthesis of copper oxide nanoparticles mediated by aqueous leaf extracts of Leucas aspera and Morinda tinctoria. Mater Lett. 10(4):2706–2714.
[Google Scholar]
- Santhoshkumar J, Kumar SV, Rajeshkumar S (2017) Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour Technol. 3(4):459–465.
[Google Scholar]
- Patra P, Mitra S, Debnath N, Pramanik P, Goswami A (2014) Ciprofloxacin conjugated zinc oxide nanoparticle: A camouflage towards multidrug resistant bacteria. Bull Mater Sci. 37(2):199–206.
[Google Scholar]
- Akpomie KG, Ghosh S, Gryzenhout M, Conradie J (2021) One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Sci Rep. 11(1):1–17.
[Crossref] [Google Scholar] [PubMed]
- Batool M, Khurshid S, Qureshi Z, Daoush WM (2021) Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem Pap. 75(3):893–907.
[Google Scholar]
Citation: Tirop DK, Njenga L, Kiplagat A, Lagat G, Onani M (2025) Characterization and Evaluation of the Antibacterial Activity of
Zinc Oxide Nanoparticles (ZnO NPs) Synthesized from the Latex of Synadenium grantii. J Nanosci Nanotechnol Res. 9:36.
Copyright: © 2025 Tirop DK, et al. This is an open-access article distributed under the terms of the Creative Commons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author
and source are credited.




