Mayank Aggarwal1 and Robert McKenna2*
1Division of Biology and Soft Matter, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
2Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL 32610, USA
Corresponding Author:
Dr. Robert McKenna
Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, USA.
Tel: 352-392-5696,
E-mail: rmckenna@ufl.edu
Received date: December 11, 2015 Accepted date: December 13, 2015 Published date: December 16, 2015
Citation: Aggarwal M, McKenna R. Carbonic Anhydrases: Nature’s Way to Balance CO2 Concentration. Biochem Mol Biol J. 2016, 1:1.
The carbonic anhydrases (CAs; EC 4.2.1.1) are a family of structurally diverse (in both fold and oligomeric state), yet efficient metalloenzymes that catalyze the reversible hydration of CO2 and bicarbonate. They are categorized into five distinct classes (α, β, γ, δ, and ζ). Among these, the αCAs are found primarily in vertebrates, the βCAs are dominantly expressed in higher plants and some prokaryotes, while γCAs are present only in archaebacteria, and the δ and ζ classes have thus far been only isolated in diatoms. These ubiquitous enzymes equilibrate the reaction between three simple chemical molecules: CO2, bicarbonate, and protons; hence, they have important roles in ion transport, acid-base regulation, gas exchange, photosynthesis, and CO2 fixation (Figures 1A-1C) [1].
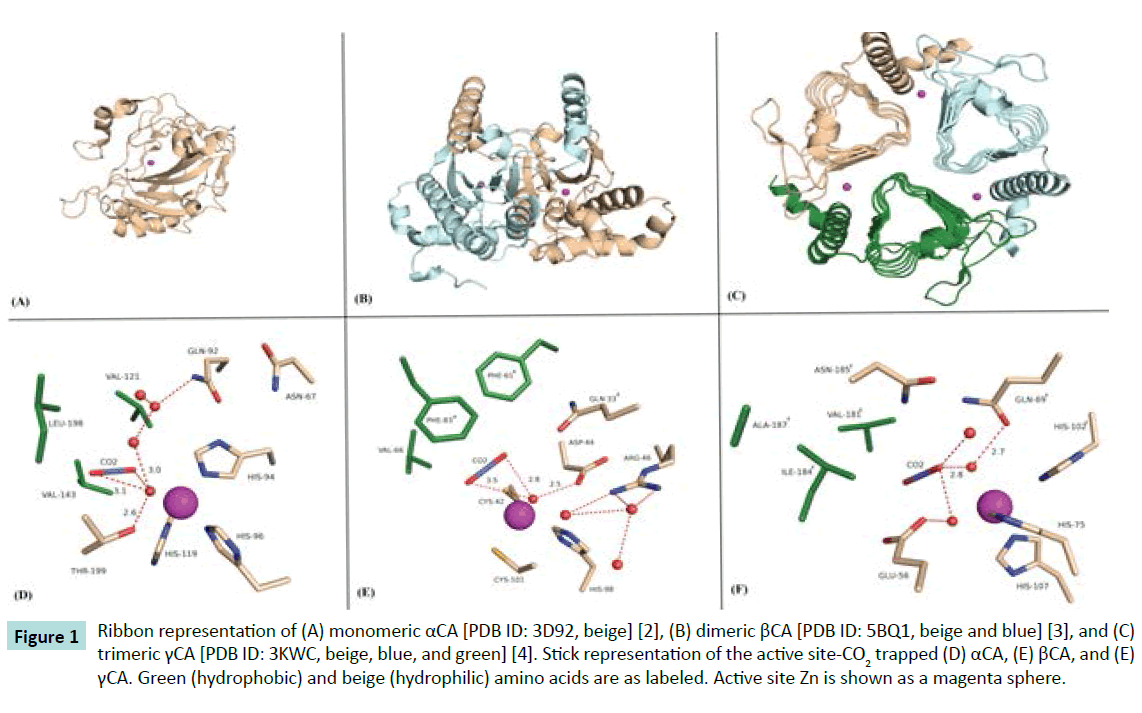
Figure 1 Ribbon representation of (A) monomeric αCA [PDB ID: 3D92, beige] [2], (B) dimeric βCA [PDB ID: 5BQ1, beige and blue] [3], and (C) trimeric γCA [PDB ID: 3KWC, beige, blue, and green] [4]. Stick representation of the active site-CO2 trapped (D) αCA, (E) βCA, and (E) γCA. Green (hydrophobic) and beige (hydrophilic) amino acids are as labeled. Active site Zn is shown as a magenta sphere.
As such, structural studies of how this family of enzyme binds CO2 and convert it to bicarbonate may help in the understanding and designing of bio-industrial technologies for carbon sequestration. Recently, high-pressure cryo-crystallography studies have been successful in “trapping” CO2 in the active sites of an αCA and a βCA (Figures 1D and 1E) [2,3]. Note, Figure 1E shows a model of a γCA-CO2 complex which is based on the structural similarities observed between the αCA and βCA-CO2 complexes.
These studies are significant for several reasons: (1) they demonstrate a substrate (with a kcat/KM approaching diffusion controlled limits of 108 M-1s-1) can be captured in an enzyme active site, (2) they show the mechanistic orientation of CO2 in a hydrophobic pocket, positioned and poised for the nucleophilic attack of a zinc-bound hydroxide to produce bicarbonate, but most importantly (3) they demonstrate that structurally distinct enzyme folds have evolutionarily converged to create very similar active sites that maintain CO2 and bicarbonate concentrations in cells [4].
Acknowledgement
This study was in part funded by National Institutes of Health grants GM25154 and CA165284.
References
- Frost SC, McKenna R (2014) Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications. Springer Science & Business Media, Netherlands.
- Domsic JF, Avvaru BS, Kim CU, Gruner SM, Agbandje-McKenna M, et al. (2008) Entrapment of carbon dioxide in the active site of carbonic anhydrase II. J BiolChem 283: 30766-30771.
- Aggarwal M, Chua TK, Pinard MA, Szebenyi DM, McKenna R (2015) Carbon Dioxide “Trapped” in a ß-Carbonic Anhydrase. Biochemistry (Mosc) 54: 6631-6638.
- Peña KL, Castel SE, de Araujo C, Espie GS, Kimber MS (2010) Structural basis of the oxidative activation of the carboxysomal gamma-carbonic anhydrase, CcmM. ProcNatlAcadSci USA 107: 2455-2460.


