Review Article - (2015) Volume 1, Issue 1
Asim Bhaumik* and Piyali Bhanja
Department of Materials Science, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, India
Corresponding Author:
Asim B
Department of Materials Science
Indian Association for the Cultivation of Science
Jadavpur, Kolkata 700032, India
Tel: +91-33-2473-4971
E-mail: msab@iacs.res.in
Advancement of human civilization is largely dependent on the use of natural fossil fuel resources and with the rapid technological development its reserve depleting very fast. To cop-up with this concern bio-refinery is an emerging and necessary approach as a substitute of primary energy sources. Liquid fuels and highly valuable fine chemicals, which are derived from petroleum resources can be produce very effectively from biomass via platform chemical 5-hydroxymethylfurfural (HMF) and this has great significance in the context of green chemistry. US department of energy has enlisted top ten high value bio-based chemicals among which HMF, furfural and 2,5-furandicarboxylic acid (FDCA) can be derived from biomass via catalytic processes. Thus the objective of the present review is to summarize various catalytic methods to produce 5-hydoxymethylfurfural and 2,5dimethylfuran from a variety of monomeric bioresources such as glucose, fructose, dimeric (sucrose) and also polymeric carbohydrates like starch, cellulose, inulin and biomass derived carbohydrate (raw biomass) for preparing liquid energy fuel. To produce these chemical and fuel artificially, porous nanomaterials have huge potential to be explored as catalyst and these materials play pivotal role in the bio-transformation processes due to their high surface acidity and porous nanostructure. Moreover, the diversity of various catalysts used for production of these energy substitutes, specific reaction mechanism, drawbacks, its economical significance are highlighted in this review. Roles of several porous catalytic materials like porous resin, micro/mesoporous carbon, microporous zeolite, mesoporous metal oxides, porous organic polymer to upgrade the selectivity of biomass conversion with yield and their thermal, hydrothermal stability and controllable acidity have also been discussed in detail in this review. Examples of new porous nanomaterials with functionalised surface in comparison to that of the conventional acidic materials have also been discussed.
Keywords
Nanomaterials, Fuel, Energy
Introduction
In past few decades modern world is mostly dependent on oil as a primary source of energy. Exponential economical growth of the developed and developing countries like India and China, the demand is likely to be increased more and more in the forthcoming years. Rapid utilization of natural fossil fuels as a greater part of energy sources since last few decades at the advent of massive civilization and industrialization resulted depletion of these reserves [1]. A new recyclable and renewable resource needs to be prepared to balance this crisis. Increased CO2 emission from the natural resource also found to be a major contributing factor of global warming and this has a devastating effect on earth’s eco-system. Owing to these serious concerns a constant effort has been devoted by researchers to seek for an alternative source of energy in the near future [2]. In recent times, there is an increased interest for biobased chemicals as non-conventional energy resources such as carbohydrates, non-food biomass, ligonocellulosic compounds, bioethanol etc. High oxygen content in the molecular structure of carbohydrates is a limitation [3]. Oxygen content can be lowered using three main pathways. First of all, by the removal of small highly oxidized carbon molecules such as CO2, formic acid and formaldehyde. Formation of ethanol, butanol and CO2 is an example of fermentative conversion of carbohydrate. Removal of oxygen from the molecule by hydrogenolysis is another method which typically removes oxygen to form water combining with one molecule of hydrogen. The third option is dehydration of carbohydrates to furans and levulinic acid. Although bioethanol serves as a fuel supplement when mixed with gasoline, it can act as a long term renewable fossil fuel alternative. In large quantities it is currently produced from grains such as corn and this is a major concern as this directly competes with the food supply [4]. Apart from that bioethanol has some serious limitation like high volatility, low energy density and contamination by moisture from the atmosphere. To produce more economical and sustainable alternative with lesser drawbacks lignocellulosic compounds have shown promising results for future perspectives [5]. In 1951, Newth [6] first published an article on furan production from carbohydrate. Since then researcher’s interest for the production of bioenergy from biomass through catalytic processes has grown gradually [7]. In 1980’s HMF production from carbohydrates were mainly based on aqueous based mineral catalysed system. The history of HMF synthesis and its real field application summarised and published by Lewkowski’s furan chemistry review in 2001 [8]. Lately, ionic liquids are used as eco-friendly solvents by Lima [9] and Stark [10] for selective sugar dehydration. Many researchers experimented HMF as an introductory compound to produce highly economical chemicals such as promising next generation polyester building block monomers (2,5-furandicarboxylic acid (FDCA), 2,5-bis(hydroxymethyl)furan (BHMF) and 2,5-bis(hydroxymethyl) tetrahydrofuran (BHMTF)) and potential biofuel candidates (2,5 dimethylfuran (DMF), 5-ethoxymethylfurfural (EMF), ethyl levulinate (EL) and γ-valerolactone (gVL)) directly from biomass via one-pot green catalytic process (Figure 1) [11-17].
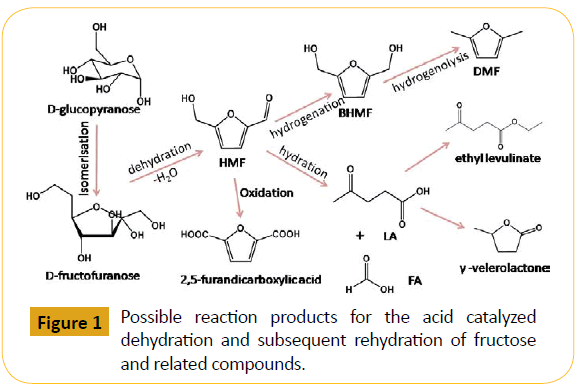
Figure 1: Possible reaction products for the acid catalyzed dehydration and subsequent rehydration of fructose and related compounds.
Lignocellulosic compounds have versatile uses and they have abundant supply mainly from agricultural industry and paper producing plants. The major constituents of these compounds are 40–50% cellulose, 16–33% hemicelluloses and 15–30% lignin, and these are available in several industrial waste streams [18]. As cellulose is the primary component, it has gained more attraction for the biomass conversion processes. Several methods are implicated for the hydrolysis of cellulose using various types of catalysts and solvents such as mesoporous carbon functionalized with metal or acid groups, ionic liquids, and supercritical water, sulfonated ion exchange resins etc., [19-23]. Degradation of cellulose breaking β-1,4-glycosidic bonds is a complicated procedure. To overcome this complicacy ionic liquids have been introduced to obtain a homogeneous solution prior to hydrolysis. Cellulose hydrolysis under some simplified condition can also yield high amount of glucose though it has several limitation like large portion of unreacted cellulose and separation of glucose from the homogeneous solution. Due to complex hydrogen bonded chemical structure present in the lignocellulosic compound requires various pre-treatment before enzymatic hydrolysis, has very narrow cost effectiveness. Using bifunctional solid catalyst Pt/γ-Al2O3 direct conversion of cellulose to sugar alcohols is possible up to a certain extent [24]. Further, conversion of amorphous cellulose into glucose using sulphonated activated carbon has been demonstrated by Onda et al with considerably high product yield [25]. Moreover, several nanocomposite materials with variable density are designed to hydrolyse the cellulose, fails to show promising results due to leaking of polycyclic aromatic hydrocarbon containing –SO3H groups [26]. As study progressed, it is possible to produce a high yield of glucose from biomass employing sulphonated mesoporous carbon [27]. Hence a detailed research has been devoted to design solid acid catalyst with substantial surface modification utilizing acidic functionalized groups to increase the product selectivity as well as efficiency for biomass degradation.
On the other hand, the application of Lewis acidic Sn-β zeolite along with aqueous HCl can convert glucose to 5-HMF at 180°C in a biphasic system with approximately 60% HMF selectivity, though corrosiveness of HCl is a limiting factor in the context of green chemical pathway [28]. Replacing the aqueous phase by N,N-dimethylformamide, solid acid resin and solid base hydrotalcites in a single reactor is also an effective procedure for this biomass conversion process [29].
The use of expensive ionic liquids (IL) as solvents and HMF separation from high boiling ionic liquid is troublesome. Water, formed during dehydration reaction deactivates the IL’s is a serious limitation and high boiling point solvents like DMSO, DMF etc are unable to resolve the drawbacks related to the separation issues [30]. High concentration of oligomeric compounds generated as a by-products in a organic solvent mediated dehydration. Therefore these methods are neither suitable nor cost effective for commercial large scale purpose. Due to the aforesaid drawbacks of monophasic solvent system using high boiling point organic system current research efforts are concentrated to utilize biphasic solvent for HMF production [31]. In the biphasic solvent system the organic part acts as a separating unit of the HMF generated subsequently. This removes the separation related problem caused in the former method, efficiently recycle the aqueous phase and due to the use of heterogeneous catalysts it can be reutilized for the next reaction. Another determining factor regarding productiveness of biphasic solvents is partition coefficient (R) which is the ratio of HMF in organic phase to that of the aqueous phase. Higher the ratio denotes more effective extraction and increases HMF selectivity. The nature of the organic solvents along with presence of inorganic salt is an additional factor determines the outcome. For an example sodium chloride (NaCl) in aqueous phase also acts to improve the partition coefficient of HMF [32]. Using biphasic reaction it is therefore possible to receive high HMF yield without any unwanted by-products, which is also cost effective and simplified extraction method. So, current attempt is to prepare improved level of biphasic solvent system to provide highly selective HMF that can be used for commercial purpose [33]. Subsequently, numerous journals articles have been published in past few years, which documented these beneficial effects of biphasic solvents for the catalytic biomass conversion from carbohydrates and lignocellulosic compounds.
In this context, various modified solid acid catalysts have been developed with a tunable pore architecture for conversion of biomass to fine important chemical like 5-hydroxymethylfurfural (HMF). However, porous metal oxides, sulphonated porous organic polymers (POPs), functionalized zeolites, alluminosillicates (ZSM- 5), immobilized ionic-liquids and acid functionalized mesoporous silica materials recently opened a new window for biomass conversion with very little pitfalls. In the next few sections we will discuss various factors associated with this reaction descriptively and also with their limitation and future scopes as well.
Biomass Conversion over Various Acid Catalysts
In the recent years, considerable progress has been made to obtain biofuels and polyester building block chemicals from HMF [34,35]. HMF is an important building block because it contains two different functionality e.g. aldehyde and hydroxyl groups which permits the various kind of chemical transformation like hydrogenolysis, oxidation and condensation reactions [36-38]. The synthesis of HMF from cellulose and different sugar derivatives are catalysed using several Lewis acidic sites present in the solid acid catalysts, mesoporous materials containing suitable Brønsted acidic sites etc [39,40]. On the other hand, for the conversion of carbohydrates into HMF several homogeneous catalyst has been used including AlCl3 in aqueous and biphasic solvents, CrII/CrIII halides in imidazolium ionic liquids, GeCl4 in 1-butyl-3- imidazolium chloride ([BMIM]Cl), SnCl4 in 1-ethyl-3-imidazolium tetrafluoroborate ([EMIM]BF4), Zr(O)Cl2/CrCl3 in DMA/LiCl (DMA = dimethylacetamide) etc., [41-44,34b]. Some homogeneous Lewis acid catalysts have been reported for the conversion of cellulose and hemicelluloses which have strong hydrogen bonded chemical structure such as CuCl2/CrCl3 and CrCl2 etc [45,46]. In recent times, the Brønsted acidic sites bearing catalysts involving N-methyl-2-pyrrolidinone methylsulfonate ([NMP][CH3SO3]) ionic liquid, N,N-dimethylacetamide methylsulfonate ([DMA][CH3SO3]) have been also mentioned to be efficacious for hydrolysis of plant biomass and dehydration of different sugar units [47,48]. Though, homogeneous catalysts are used in some industries like food, fine chemicals, pharmaceuticals, agrochemicals etc. But today, heterogeneous catalysis has been proved to be the ultimate goal of industrial catalysis and engineering field due to increasing public awareness regarding environmental issues and drawbacks associated with the homogeneous catalysts with respect to the difficulty of catalyst separation, its recovery, regeneration and reuse, corrosions, toxicity, creation of huge solid waste etc., [49]. In Figure 1 we have illustrated the synthesis of different value added chemicals and biofuels from various carbohydrates. The possible mechanistic pathway for HMF production from cellulose is illustrated in Figure 2.
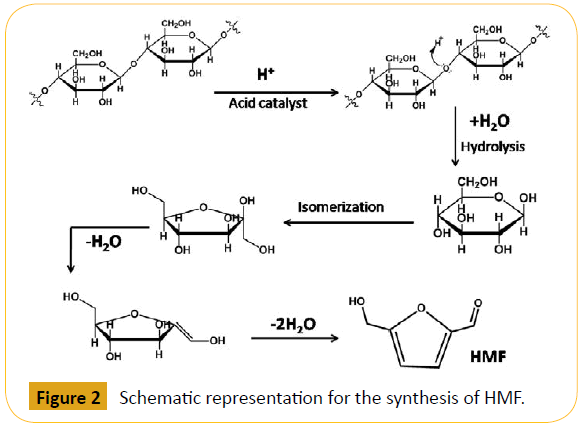
Figure 2: Schematic representation for the synthesis of HMF.
Mesoporous silica
Mesoporous materials having large BET surface area, pore volume with tunable pore diameters are extensively used in various application areas like gas adsorption, catalysis, optical densities, sensing etc [50-53]. Materials with functionalized pores bearing organic groups e.g. –SO3H, –COOH and uniformly distributed pore size are important contenders within the mesoporous family of materials. Among the mesoporous silica material SBA-15 materials are more acceptable in comparison to MCM-41 due to their large pore diameter, hydrothermal and mechanical stability [54]. Owing to high BET surface area and tunable pore size ordered mesoporous silicas (MCM-41 and SBA- 15) have contributed significantly in the field of heterogeneous catalysis. The silica based materials contains high concentration of silanol groups actually acts bridge for covalent immobilization of active sites, also provides additional organic functionality to enable for controlling surface hydrophobicity to enhance their catalytic performance like durability, activity and selectivity in various acid catalyzed chemical reactions. The active acid sites of these functionalized ordered mesoporous silica materials are very attractive in the context of biomass conversion. Dufaud and Davis in 2003 synthesized mesoporous silicas with numerous active sites such as sulphonated acid using organic-inorganic hybrid silane [54]. It has been observed that the sulphonic acid group grafted on periodic mesoporous organosilane (PMO) with a proper density of additional organic functional groups offer versatile solid acids that allows to achieve high degree of selectivity in the biphasis (organic : aqueous) solvent for dehydration of fructose to HMF by adjusting the surface hydrophilicity and hydrophobicity of the catalyst [55]. SBA type mesoporous silica supported sulphonic acid sites (SBA-SO3H) found to be efficacious in catalyzing the dehydration of fructose in a water-methyl isobutyl ketone-2-butanol biphasic solvent to convert HMF with the yield of 60% [56]. However, in-spite of the high yield product, it has been noted the catalyst is deactivated in water due to leaching of active sites and a strong solvation. Mesoporous-macroporous SBA-15 sulphonic acid catalyst produced by Dhainaut et al. with polystyrene as a starting gel, are more efficient than conventional sulphonated mesoporous SBA-15 in transesterification of tricaprylin and esterificaton of palmitic acid using methanol [57]. Karimi et al has reported the high HMF yield for dehydration of fructose over two different ordered mesoporous solid acid catalysts in different solvents. The HMF selectivity (and of course the HMF yield) was gradually improvised to achieve a maximum value of 75% selectivity at the 93% fructose conversion in water-organic biphasic solvent system (∼70% HMF yield) over SBA-15-PrSO3H [58].
Bifunctional catalyst
For initiating serial cascade reactions in the biomass conversion process it is necessary for a catalyst to have bifunctional properties like acidic and basic characters. Materials containing functionalized carbon, zirconia, titania found to have high thermal stability indeed essential for application like biomass conversion. HMF production by combining both acid and base functionalized mesoporous silica demonstrated by Peng et al in an ionic liquid resulted approximately 0.54 mol HMF per mol catalyst per h at 393 K temperature [59]. 3-((3-(trimethoxysilylpropyl)thio) propane-1-sulfonic acid (TESAS) when functionalized at the surface of SBA-15 material, it showed quite high yield of HMF (71%) and selectivity of 84% due to presence of thiol group which imparts significant hydrophobic nature [56]. Wilson et al have used a bifunctional sulphated zirconia (SZ) catalyst for aqueous phase conversion of glucose to HMF. In this reaction the acid base properties of the catalyst have been modified by utilizing amphoteric nature of zirconia with controlled surface sulphonation [60]. They have also reported a synthetic route for high surface area SZ/SBA-15 catalysts having a balance of surface base/Lewis acid sites (to drive isomerization to fructose) and Brønsted acid sites (for the subsequent dehydration) [61]. Fu and co-workers [62] have combined sulphonated mesoporous silica (SBA-15) and magnetic iron oxide nanoparticle Fe3O4 to produce a magnetically separable solid acid catalyst which showed high yield of glucose via cellulose hydrolysis in the aqueous medium. Mazzotta et al. [63] have mentioned the catalytic effectiveness of solid acid catalyst containing Lewis and Brønsted acid sites (Glu- TsOH-Ti) for the dehydration of fructose, glucose, and cellobiose in the MeTHF/H2O biphasic solvent with maximum yields of 59, 48, and 39%, respectively. Figure 3 depicts the possible dual role of the Brønsted and Lewis acid sites for the facile biomass conversion.
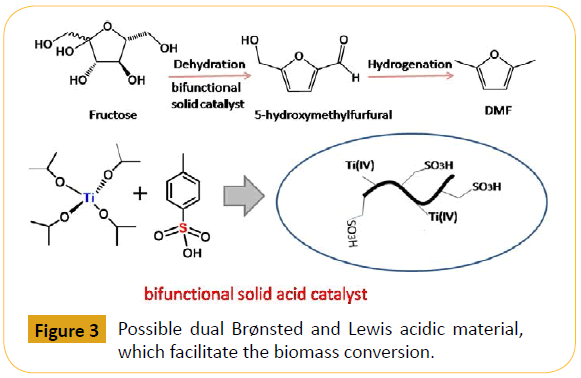
Figure 3: Possible dual Brønsted and Lewis acidic material, which facilitate the biomass conversion.
Porous metal oxides
Using soft-templates like aspartic acid and salicylic acid mesopores are introduced at the surface of the metal oxide materials, and they can efficiently converts glucose and fructose to HMF [64,65]. As because the surfaces of the metal oxides bearing hydroxyl group have a very little Brønsted acidity, the Brønsted acid strength of the resulting material is increased by sulphonation in which sulphuric acid groups with S=O bond are avidly bonded to metal atoms constituting the S=O bond with hydroxyl group on metal oxide. Biopolymers can be used as template to design porous TiO2 material, which displayed high catalytic efficiency for the conversion of unutilized hemicellulosic sugars like galactose, lactose to HMF [66]. Dutta et al. [64] have reported the selfassembled mesoporous spherical nanoparticulate TiO2 material, which can be employed as solid catalyst for the microwaveassisted conversion of carbohydrates into HMF in aqueous and organic media, yielding 34.3% and 54.1% HMF in water and DMSO, respectively [64]. The biopolymer in the catalyst containing alginate acts as a template to introduce large pores of nanoscale dimensions at the material surface and this is responsible for high catalytic activity. De et al. [67] have successfully prepared porous TiO2 nanoparticulate catalysts via biopolymer alginate templating pathway under hydrothermal conditions and this material can catalyze the transformation of unutilized sugar derivatives like d-mannose, d-galactose, and lactose to useful platform chemical HMF in DMA-LiCl under microwave irradiation at 140°C, which produced maximum 44% yield.
Due to lower electronegativity of Sn and Zr metals than Ti metal protons are released more easily, hence SO4 2-/SnO2 and SO4 2-/ ZrO2 showed stronger acidity than SO4 2-/TiO2. Low surface area of sulphated nanoporous oxides prevents the access of active acid sites to reactants. Jimenez-lopez et al. [68] have synthesized supported SO4 2-/ZrO2 in mesoporous silica material (Zr-MCM) and it showed high yield of ethyl ester via ethanolysis of sunflower oil.
Mixed oxides
Today 2,5-furandicarboxylic acid (FDCA) is regarded as an essential biomass derived chemical building block agent with high economic potential as it can replace terephthalic acid and PET (polyethylene terephthalate) manufacture. Neatu et al. [69] have demonstrated an eco-friendly and environmentally sound method to synthesis FDCA. Yang et al illustrated a process for the degradation of cotton cellulose in the presence of mixed metaloxide ZnO–ZrO2 catalyst under the mild hydrothermal conditions (463 K, 1.4 MPa) for the synthesis of HMF. The conversion ratio of cotton cellulose to HMF is reached 81% at 483 K temperature [70]. Hora et al have reported furfural conversion at approximately 100% condensation of furfural with acetone over Mg–Al based HT [71].
Porous carbon
Sulfonated carbons are much more efficacious in comparison to resin and oxide type solid acid catalysts due to preferable texture. To enhance hydrolysis of cellulose surface acidity and hydrophobicity/htdrophilicity is tuned by sulphonation under suitable conditions. Hence nanoporous carbon supported acidic sites or metal nanoparticles are efficiently utilized as catalyst for the biomass conversion utilizing this principle. Very recently Zhang et al. [72] have utilized sulphonated mesoporous carbon in the cellulose hydrolysis reaction for the synthesis of glucose in high yield (75%). Chang et al. [73] in other way pretreated the surface of ordered mesoporous carbon with H2O2 to introduce high amount of hydroxyl and carboxyl groups. After that sulphonation with concentrated sulphuric acid produced OMC-H2O2–SO3H material, which showed much higher acid concentration of 2.09 mmol/g than conventional sulphonated samples (OMC-SO3H) [74]. Hara et al. [75] thoroughly described sulphonated activated carbon, hydrolysing cellulose completely to β-1,4-glucans at 100°C. In this context nickel phosphide supported over activated carbon acts as a catalyst to produce sorbitol with moderate yield of 48% [76].
Zeolite
The petrochemical and fine industrial plants utilize zeolite crystals as a principle heterogeneous catalyst for more than four decades. Zeolites have both strong Brønsted and Lewis acid sites with properly arranged micropores, extraordinary thermal stability due to crystalline inorganic framework. However, zeolites are sensitive to hot water, which leads to limit its utilization in aqueous phase process like upgrading reactions and biomass conversion. The active acidic sites of zeolite (H-ZSM) acts as a carbonium ion and effectively used in the pyrolysis of wood biomass [77]. A corroborative study regarding cellulose hydrolysis with solid acid catalyst like H form zeolite with variable Si/Al ratio, sulphated zirconia, sulphated activated carbon and ion-exchange resin under proper hydrothermal conditions (423 K) suggested increased glucose selectivity for zeolite catalyst with higher Si/Al ratio (H-beta (rSi: Al=75), H-ZSM-5 (rSi: Al=45)) than that of the zeolite with lower Si/Al ratio (H-beta (rSi: Al=13) and H-mordenite (rSi: Al=10)) [78]. The plausible explanation of relatively high glucose yield could be due to high hydrophobic character of zeolite with high Si/Al ratio.
Owing to shape selectivity today zeolites are extensively employed as solid acid catalyst for various industrial purposes [79]. The crystalline microporous alluminosilicates (zeolite) having polar Si-O bond and ionic charges are able to introduce relatively strong electric field when applied for any chemical reaction. Lercher and co-workers invented [80] advancement in the pyrolysis of oil using highly active and recyclable Ni or Pd catalysts with alluminosillicate ZSM-5 and beta zeolite. It has been observed that higher yield of aromatics in first pyrolysis of glucose can be achieved when small micropored zeolites (4.0–5.9 Å) like ZSM-5, ZSM-11, ZSM-23, TNU-9, MCM-22 and IM-5 are employed [81]. HMF can be obtained from glucose, cellobiose and starch through one-pot reaction employing a mixed catalyst of Sn-Beta and hydrochloric acid with a biphasic solvent to extract the formed HMF subsequently from aqueous phase to organic phase and also preventing its hydration in the former phase [82]. It is noteworthy that both hydrogenation and etherification of 5-hydroxymethyl furfural with primary and secondary alcohols can be carried out over Hf- , Zr- and Sn- Beta zeolites as a catalyst in absence of any external hydrogen source or expensive metal for the synthesis of biofuels.
In North America, a huge reserve of natural gas has been found recently which increased the interest to introduce a proper, simplified, economical and viable method to convert cheap energy source to highly precious fuel source. The efficient and productive methodology regarding conversion of methane to complex hydrocarbon is not very satisfactory. Wang et al. in 1993 discovered a feasible method of conversion of methane to benzene through a process of dehydrogenation and aromatization with 100% selectivity using Mo and Zn catalyst loaded with ZSM-5 under inert atmosphere [83].
Sulphonated resin
Now-a-days there is a growing interest for using commercially synthesized sulphonated resin such as Amberlyst, EBD resins, and Nafion for the conversion of biomass to bioenergy [84-86]. Polymerization of styrene followed by its sulphonation resulted the sulphonated Amberlyst-15 resin and it is the most well-known resins with rich macropores and mesopores with a very high acidity (4.0 mmol g−1). Sulphonated amberlyst-15 displayed 100% HMF yield for fructose conversion to HMF in a water separation reactor [87].
Metal-organic Frameworks (MOFs)
Owing to high BET surface area, modifiable microarchitecture and tunable pore diameter have made metalorganic frameworks (MOFs) very demanding in the context of heterogeneous catalyst. Hence, researchers have tried MOF derived solid acids as a heterogeneous catalyst for biotransformation of carbohydrate to HMF. Through post-synthetic modification the functional group in the MOF containing organic ligand component adds a beneficial effect than conventional carbon materials and inorganic solids [88]. In addition to that, more substrate transfer within the MOF catalyst is possible due to highly porous and ordered nature of the catalyst. Li and Hensen [89] mentioned a selective dehydration of fructose to HMF using MOF and phosphotungstic acid (PTA)-encapsulated MIL-101(Cr), [PTA/MIL-101 (Cr)], as a solid acid catalyst.
A recent study suggested one-pot conversion of cellobiose and cellulose into sorbitol using a bi-functional acid metal catalyst [Ru-PTA/MIL-100 (Cr)] containing ruthenium and PTA as active species with a MIL-100 (Cr) as support and encapsulation matrix [90]. Fructose transformation to HMF is possible utilizing a series of sulphonic acid functionalised metal-organic framework (MOF-SO3H) including MIL-101 (Cr) [MIL-101 (Cr)-SO3H], UIO-66 (Zr) [UIO-66 (Zr)-SO3H], and MIL- 53 (Al) [MIL-53(Al)-SO3H]. When MIL-101 (Cr)-SO3H is used as a catalyst a HMF yield of 90% is obtained with a full fructose conversion [91]. Cirujano et al. studied [92] levulinic acid esterification using various alcohols including ethanol and butanol. They have shown that zirconium containing UiO-66 and UiO-66–NH2 materials can act as a very efficient catalyst for this reaction. Bromberg et al. [93] described a simple and direct methodology to synthesize novel functional composite materials that are building block of MOF and polymer network. These agents are highly efficient for feasible synthesis of highly important chemical intermediates through dehydration of fructose from renewable resources.
Metal phosphate
Metal phosphates are a specific class of catalyst, showing very promising results for various dehydration reactions [94]. When NbPO, AlPO, TiPO and ZrPO were applied in glucose dehydration to HMF in aqueous phase, they showed high catalytic activity and their activity largely depends on the amount of strong acid sites present in the catalyst.95 Zhang et al demonstrated96 another method for fructose dehydration to achieve high yield of HMF (~71%) over tin phosphate in the presence of a mixed solvent of water-DMSO at temperature of 135°C for 1 h. Zirconium and titanium phosphates can catalyze the reaction like alcohol dehydration and olefin isomerisation. Benvenuti et al. [97] have reported the cubic zirconium pyrophosphate, which showed very high yield of HMF from carbohydrate biomass. Carlini et al. [98] have showed selective oxidation of 5-hydroxymethyl- 2-furaldehyde to furan-2,5-dicarboxaldehyde over vanadyl phosphate based catalyst under variable reaction conditions like reaction temperature, type of the solvent (water, organic) and oxidizing agent (air, oxygen and their pressure). Dutta et al. demonstrated a method of excellent catalytic activity for the transformation of fructose, glucose, sucrose, cellobiose and cellulose to 5-hydroxymethylfurfural (HMF) in water/methyl isobutyl ketone (MIBK) biphasic solvent to give maximum yields of 77, 50, 51, 39, and 32 mol%, respectively, under microwaveassisted heating conditions at 423 K over large-pore mesoporous self-aggregated tin phosphate nanoparticles (LPSnP-1) catalyst [99]. The schematic representation for the formation of LPSnP-1 catalyst is shown in Figure 4, where size of the mesopore plays crucial role in the overall HMF yield.
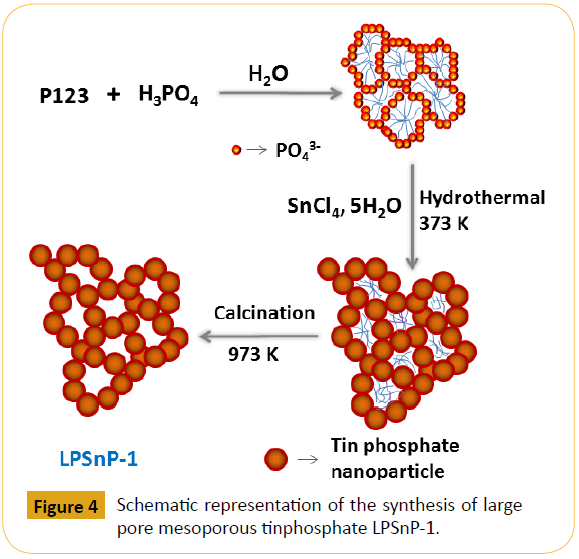
Figure 4: Schematic representation of the synthesis of large pore mesoporous tinphosphate LPSnP-1.
Future Perspectives and Conclusion
Although in recent times extensive fundamental research and experiments are carried out to optimize the HMF synthesis, commercial HMF production is still a challenge on economic point of view. The major limitation in this context is the absence of an effective and scalable system for dehydration of carbohydrates from glucose and glucose based polysaccharides. Despite high yields of HMF obtained by using ionic liquid or high boiling point polar aprotic medium with chromium catalyst, these systems are not suitable for large scale production due to toxicity of the process. Hence, more and more experiments, observations, calculations indeed required for an environmentally sound, economical, sustainable and purposeful procedure for the dehydration of glucose based carbohydrates. The Life cycle analysis (LCA) is an approach as per sustainability of biomass conversion process is concerned, which precludes assessment of all inputs and outputs of production system (processing, manufacture, distribution, use and maintenance, and disposal or recycling). Instead of this, the raw materials, catalysts, chemical procedure involved in biomass conversion barely follows the approach. For practical point of view the window of application, its scope in near future and economical value has made HMF an investigative tool in current research work. Furthermore, implementation of proper methodology of HMF production, application of HMF and its derivatives must be efficacious and advantageous than petroleum based platform chemicals. In near future HMF derivatives can be used in medical application too. To sum up, utilization of unused raw materials for production of HMF as bio-renewable energy source will lead us to a greener, safer and sustainable future for centuries.
Acknowledgements
PB thanks CSIR, New Delhi for a junior research fellowship. AB wishes to thank DST, New Delhi for instrumental facilities through DST Unit on Nanoscience, DST-SERB and DST-UKIERI project grants.